当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antibody-Free Targeted Proteomics Assay for Absolute Measurement of α-Tubulin Acetylation.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-07-08 , DOI: 10.1021/acs.analchem.0c01683 Alok K Shah 1 , Gautam Wali 2 , Carolyn M Sue 2 , Alan Mackay-Sim 2, 3 , Michelle M Hill 1, 4
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-07-08 , DOI: 10.1021/acs.analchem.0c01683 Alok K Shah 1 , Gautam Wali 2 , Carolyn M Sue 2 , Alan Mackay-Sim 2, 3 , Michelle M Hill 1, 4
Affiliation
|
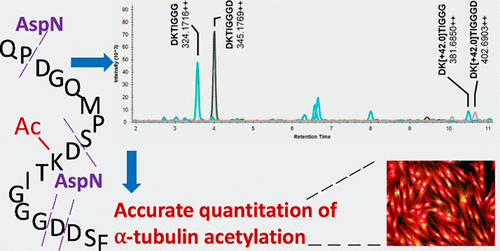
Acetylation of α-tubulin at conserved lysine 40 (K40) amino acid residue regulates microtubule dynamics and controls a wide range of cellular activities. Dysregulated microtubule dynamics characterized by differential α-tubulin acetylation is a hallmark of cancer, neurodegeneration, and other complex disorders. Hence, accurate quantitation of α-tubulin acetylation is required in human disease and animal model studies. We developed a novel antibody-free proteomics assay to measure α-tubulin acetylation targeting protease AspN-generated peptides harboring K40 site. Using the synthetic unmodified and acetylated stable isotope labeled peptides DKTIGGG and DKTIGGGD, we demonstrate assay linearity across 4 log magnitude and reproducibility of <10% coefficient of variation. The assay accuracy was validated by titration of 10–80% mixture of acetylated/nonacetylated α-tubulin peptides in the background of human olfactory neurosphere-derived stem (ONS) cell matrix. Furthermore, in agreement with antibody-based high content microscopy analysis, the targeted proteomics assay reported an induction of α-tubulin K40 acetylation upon Trichostatin A stimulation of ONS cells. Independently, we found 35.99% and 16.11% α-tubulin acetylation for mouse spinal cord and brain homogenate tissue, respectively, as measured by our assay. In conclusion, this simple, antibody-free proteomics assay enables quantitation of α-tubulin acetylation, and is applicable across various fields of biology and medicine.
中文翻译:

绝对测量α-管蛋白乙酰化的无抗体靶向蛋白质组学分析。
保守的赖氨酸40(K40)氨基酸残基处的α-微管蛋白乙酰化可调节微管动力学并控制广泛的细胞活性。以微分的α-微管蛋白乙酰化为特征的微管动力学失调是癌症,神经变性和其他复杂疾病的标志。因此,在人类疾病和动物模型研究中,需要准确定量α-微管蛋白的乙酰化。我们开发了一种新型的无抗体蛋白质组学测定方法,以测量靶向蛋白酶AspN生成的具有K40位点的肽的α-微管蛋白乙酰化。使用合成的未修饰和乙酰化的稳定同位素标记的肽DKTIGGG和DKTIGGGD,我们证明了4 log log范围内的测定线性和变异系数<10%的可重复性。在人嗅觉神经球来源的干细胞(ONS)背景下,通过滴定10-80%乙酰化/非乙酰化α-微管蛋白肽混合物来验证测定的准确性。此外,与基于抗体的高含量显微镜分析相一致,靶向蛋白质组学分析报告了在Trichostatin A刺激ONS细胞后诱导α-微管蛋白K40乙酰化。独立地,通过我们的测定,我们发现小鼠脊髓和脑匀浆组织的α-微管蛋白乙酰化分别为35.99%和16.11%。总之,这种简单,无抗体的蛋白质组学测定方法能够定量检测α-微管蛋白的乙酰化程度,可应用于生物学和医学的各个领域。与基于抗体的高含量显微镜分析相一致,靶向蛋白质组学分析报告了在Trichostatin A刺激ONS细胞后诱导α-微管蛋白K40乙酰化。独立地,通过我们的测定,我们发现小鼠脊髓和脑匀浆组织的α-微管蛋白乙酰化分别为35.99%和16.11%。总之,这种简单,无抗体的蛋白质组学测定方法能够定量检测α-微管蛋白的乙酰化程度,可应用于生物学和医学的各个领域。与基于抗体的高含量显微镜分析相一致,靶向蛋白质组学分析报告了在Trichostatin A刺激ONS细胞后诱导α-微管蛋白K40乙酰化。独立地,通过我们的测定,我们发现小鼠脊髓和脑匀浆组织的α-微管蛋白乙酰化分别为35.99%和16.11%。总之,这种简单,无抗体的蛋白质组学测定方法能够定量检测α-微管蛋白的乙酰化程度,可应用于生物学和医学的各个领域。
更新日期:2020-08-18
中文翻译:

绝对测量α-管蛋白乙酰化的无抗体靶向蛋白质组学分析。
保守的赖氨酸40(K40)氨基酸残基处的α-微管蛋白乙酰化可调节微管动力学并控制广泛的细胞活性。以微分的α-微管蛋白乙酰化为特征的微管动力学失调是癌症,神经变性和其他复杂疾病的标志。因此,在人类疾病和动物模型研究中,需要准确定量α-微管蛋白的乙酰化。我们开发了一种新型的无抗体蛋白质组学测定方法,以测量靶向蛋白酶AspN生成的具有K40位点的肽的α-微管蛋白乙酰化。使用合成的未修饰和乙酰化的稳定同位素标记的肽DKTIGGG和DKTIGGGD,我们证明了4 log log范围内的测定线性和变异系数<10%的可重复性。在人嗅觉神经球来源的干细胞(ONS)背景下,通过滴定10-80%乙酰化/非乙酰化α-微管蛋白肽混合物来验证测定的准确性。此外,与基于抗体的高含量显微镜分析相一致,靶向蛋白质组学分析报告了在Trichostatin A刺激ONS细胞后诱导α-微管蛋白K40乙酰化。独立地,通过我们的测定,我们发现小鼠脊髓和脑匀浆组织的α-微管蛋白乙酰化分别为35.99%和16.11%。总之,这种简单,无抗体的蛋白质组学测定方法能够定量检测α-微管蛋白的乙酰化程度,可应用于生物学和医学的各个领域。与基于抗体的高含量显微镜分析相一致,靶向蛋白质组学分析报告了在Trichostatin A刺激ONS细胞后诱导α-微管蛋白K40乙酰化。独立地,通过我们的测定,我们发现小鼠脊髓和脑匀浆组织的α-微管蛋白乙酰化分别为35.99%和16.11%。总之,这种简单,无抗体的蛋白质组学测定方法能够定量检测α-微管蛋白的乙酰化程度,可应用于生物学和医学的各个领域。与基于抗体的高含量显微镜分析相一致,靶向蛋白质组学分析报告了在Trichostatin A刺激ONS细胞后诱导α-微管蛋白K40乙酰化。独立地,通过我们的测定,我们发现小鼠脊髓和脑匀浆组织的α-微管蛋白乙酰化分别为35.99%和16.11%。总之,这种简单,无抗体的蛋白质组学测定方法能够定量检测α-微管蛋白的乙酰化程度,可应用于生物学和医学的各个领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号