Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Analysis of Weakly Bound Proteins Reveals Molecular Basis of Soft Corona Formation.
ACS Nano ( IF 15.8 ) Pub Date : 2020-07-07 , DOI: 10.1021/acsnano.0c04165 Daniel Sanchez-Guzman 1 , Gaël Giraudon-Colas 2 , Laurent Marichal 3 , Yves Boulard 4 , Frank Wien 5 , Jéril Degrouard 3 , Armelle Baeza-Squiban 1 , Serge Pin 2 , Jean Philippe Renault 2 , Stéphanie Devineau 1
ACS Nano ( IF 15.8 ) Pub Date : 2020-07-07 , DOI: 10.1021/acsnano.0c04165 Daniel Sanchez-Guzman 1 , Gaël Giraudon-Colas 2 , Laurent Marichal 3 , Yves Boulard 4 , Frank Wien 5 , Jéril Degrouard 3 , Armelle Baeza-Squiban 1 , Serge Pin 2 , Jean Philippe Renault 2 , Stéphanie Devineau 1
Affiliation
|
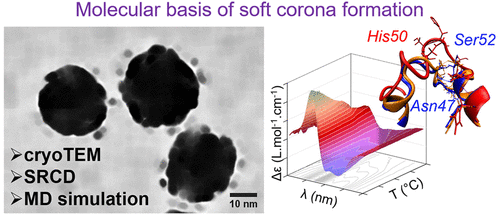
Few experimental techniques allow the analysis of the protein corona in situ. As a result, little is known on the effects of nanoparticles on weakly bound proteins that form the soft corona. Despite its biological importance, our understanding of the molecular bases driving its formation is limited. Here, we show that hemoglobin can form either a hard or a soft corona on silica nanoparticles depending on the pH conditions. Using cryoTEM and synchrotron-radiation circular dichroism, we show that nanoparticles alter the structure and the stability of weakly bound proteins in situ. Molecular dynamics simulation identified the structural elements driving protein–nanoparticle interaction. Based on thermodynamic analysis, we show that nanoparticles stabilize partially unfolded protein conformations by enthalpy-driven molecular interactions. We suggest that nanoparticles alter weakly bound proteins by shifting the equilibrium toward the unfolded states at physiological temperature. We show that the classical approach based on nanoparticle separation from the biological medium fails to detect destabilization of weakly bound proteins, and therefore cannot be used to fully predict the biological effects of nanomaterials in situ.
中文翻译:

弱结合蛋白的原位分析揭示了软电晕形成的分子基础。
很少有实验技术可以对蛋白质电晕原位进行分析。结果,对于纳米粒子对形成软电晕的弱结合蛋白的作用了解甚少。尽管它具有生物学重要性,但我们对驱动其形成的分子基础的理解是有限的。在这里,我们显示血红蛋白可以根据pH条件在二氧化硅纳米颗粒上形成硬或软电晕。使用cryoTEM和同步辐射圆二色性,我们表明纳米粒子改变了原位弱结合蛋白的结构和稳定性。分子动力学模拟确定了驱动蛋白质与纳米粒子相互作用的结构要素。基于热力学分析,我们表明纳米粒子通过焓驱动的分子相互作用稳定了部分展开的蛋白质构象。我们建议纳米粒子通过将平衡移向生理温度下的未折叠状态来改变弱结合的蛋白质。我们表明,基于从所述生物介质纳米颗粒分离的经典方法未能检测到弱结合蛋白的去稳定化,并因此不能被用于完全预测纳米材料的生物效应原位。
更新日期:2020-07-28
中文翻译:

弱结合蛋白的原位分析揭示了软电晕形成的分子基础。
很少有实验技术可以对蛋白质电晕原位进行分析。结果,对于纳米粒子对形成软电晕的弱结合蛋白的作用了解甚少。尽管它具有生物学重要性,但我们对驱动其形成的分子基础的理解是有限的。在这里,我们显示血红蛋白可以根据pH条件在二氧化硅纳米颗粒上形成硬或软电晕。使用cryoTEM和同步辐射圆二色性,我们表明纳米粒子改变了原位弱结合蛋白的结构和稳定性。分子动力学模拟确定了驱动蛋白质与纳米粒子相互作用的结构要素。基于热力学分析,我们表明纳米粒子通过焓驱动的分子相互作用稳定了部分展开的蛋白质构象。我们建议纳米粒子通过将平衡移向生理温度下的未折叠状态来改变弱结合的蛋白质。我们表明,基于从所述生物介质纳米颗粒分离的经典方法未能检测到弱结合蛋白的去稳定化,并因此不能被用于完全预测纳米材料的生物效应原位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号