当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen-Based Anion Redox for Lithium Batteries.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.accounts.0c00104 Matthew Li 1, 2 , Xuanxuan Bi 1 , Khalil Amine 1 , Jun Lu 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.accounts.0c00104 Matthew Li 1, 2 , Xuanxuan Bi 1 , Khalil Amine 1 , Jun Lu 1
Affiliation
|
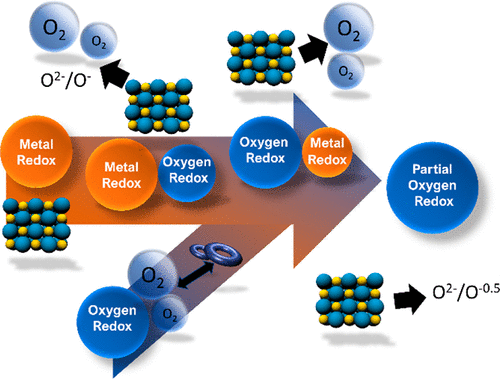
The importance of current Li-ion batteries (LIBs) in modern society cannot be overstated. While the energy demands of devices increase, the corresponding enhancements in energy density of battery technologies are highly sought after. Currently, many different battery concepts, such as Li–S and metal–air among many others, have been investigated. However, their practical implementation has mostly been restricted to the prototyping stage. In fact, most of these technologies require rework of existing Li-ion battery manufacturing facilities and will naturally incur resistance to change from industry. For this reason, one specifically attractive technology, anionic redox in transition metal oxides, has gained much attention in the recent years. Its ability to be directly used in already established processes and higher energy density with similar electrolyte formulation make it a key materials research direction for next generation Li-ion batteries. In regular LIBs, the redox active centers are the transition metal cation. In anion redox, both the anion (typically O) and the transition metal cation are utilized as redox centers with enormous implications for increasing energy density. This new material can be highly competitive for replacing the current LIB technologies. However, much is still unknown about its cycling mechanism. Upon activating the O redox couples, most cationic and anionic redox active materials will either evolve O2 or undergo irreversible structural degradation with associated severe decreases in electrochemical performance. By understanding the transition from full anion redox to partial cationic and anionic redox, we hope readers can gain a deeper understanding of the topic.
中文翻译:

锂电池的基于氧的阴离子氧化还原。
当前的锂离子电池(LIB)在现代社会中的重要性不可低估。在设备的能量需求增加的同时,人们强烈寻求电池技术的能量密度的相应提高。目前,已经研究了许多不同的电池概念,例如Li-S和金属-空气。但是,它们的实际实施主要限于原型阶段。实际上,大多数这些技术都需要对现有的锂离子电池制造设施进行改造,并且自然会招致行业变革的阻力。由于这个原因,近年来,一种特别吸引人的技术,即过渡金属氧化物中的阴离子氧化还原备受关注。它具有直接用于已经建立的过程的能力以及具有类似电解质配方的更高能量密度,使其成为下一代锂离子电池材料研究的关键方向。在常规LIB中,氧化还原活性中心是过渡金属阳离子。在阴离子氧化还原中,阴离子(通常为O)和过渡金属阳离子均被用作氧化还原中心,这对提高能量密度具有重大意义。这种新材料在取代当前的LIB技术方面具有很高的竞争力。然而,关于它的循环机制仍然很多未知。激活O氧化还原对后,大多数阳离子和阴离子氧化还原活性物质都会释放O 氧化还原活性中心是过渡金属阳离子。在阴离子氧化还原中,阴离子(通常为O)和过渡金属阳离子均被用作氧化还原中心,这对提高能量密度具有重大意义。这种新材料在取代当前的LIB技术方面具有很高的竞争力。然而,关于它的循环机制仍然很多未知。激活O氧化还原对后,大多数阳离子和阴离子氧化还原活性物质都会释放O 氧化还原活性中心是过渡金属阳离子。在阴离子氧化还原中,阴离子(通常为O)和过渡金属阳离子均被用作氧化还原中心,这对提高能量密度具有重大意义。这种新材料在取代当前的LIB技术方面具有很高的竞争力。然而,关于它的循环机制仍然很多未知。激活O氧化还原对后,大多数阳离子和阴离子氧化还原活性物质都会释放O2或经历不可逆的结构退化,伴随着电化学性能的严重降低。通过理解从完整的阴离子氧化还原到部分阳离子和阴离子氧化还原的过渡,我们希望读者可以对该主题有更深入的了解。
更新日期:2020-08-18
中文翻译:

锂电池的基于氧的阴离子氧化还原。
当前的锂离子电池(LIB)在现代社会中的重要性不可低估。在设备的能量需求增加的同时,人们强烈寻求电池技术的能量密度的相应提高。目前,已经研究了许多不同的电池概念,例如Li-S和金属-空气。但是,它们的实际实施主要限于原型阶段。实际上,大多数这些技术都需要对现有的锂离子电池制造设施进行改造,并且自然会招致行业变革的阻力。由于这个原因,近年来,一种特别吸引人的技术,即过渡金属氧化物中的阴离子氧化还原备受关注。它具有直接用于已经建立的过程的能力以及具有类似电解质配方的更高能量密度,使其成为下一代锂离子电池材料研究的关键方向。在常规LIB中,氧化还原活性中心是过渡金属阳离子。在阴离子氧化还原中,阴离子(通常为O)和过渡金属阳离子均被用作氧化还原中心,这对提高能量密度具有重大意义。这种新材料在取代当前的LIB技术方面具有很高的竞争力。然而,关于它的循环机制仍然很多未知。激活O氧化还原对后,大多数阳离子和阴离子氧化还原活性物质都会释放O 氧化还原活性中心是过渡金属阳离子。在阴离子氧化还原中,阴离子(通常为O)和过渡金属阳离子均被用作氧化还原中心,这对提高能量密度具有重大意义。这种新材料在取代当前的LIB技术方面具有很高的竞争力。然而,关于它的循环机制仍然很多未知。激活O氧化还原对后,大多数阳离子和阴离子氧化还原活性物质都会释放O 氧化还原活性中心是过渡金属阳离子。在阴离子氧化还原中,阴离子(通常为O)和过渡金属阳离子均被用作氧化还原中心,这对提高能量密度具有重大意义。这种新材料在取代当前的LIB技术方面具有很高的竞争力。然而,关于它的循环机制仍然很多未知。激活O氧化还原对后,大多数阳离子和阴离子氧化还原活性物质都会释放O2或经历不可逆的结构退化,伴随着电化学性能的严重降低。通过理解从完整的阴离子氧化还原到部分阳离子和阴离子氧化还原的过渡,我们希望读者可以对该主题有更深入的了解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号