Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The secondary‐structured DNA‐binding activity of Dna2 endonuclease/helicase is critical to cell growth under replication stress
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-07-07 , DOI: 10.1111/febs.15475 Soyeong Park 1 , Nargis Karatayeva 1 , Annie Albert Demin 1 , Palinda Ruvan Munashingha 1 , Yeon-Soo Seo 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-07-07 , DOI: 10.1111/febs.15475 Soyeong Park 1 , Nargis Karatayeva 1 , Annie Albert Demin 1 , Palinda Ruvan Munashingha 1 , Yeon-Soo Seo 1
Affiliation
|
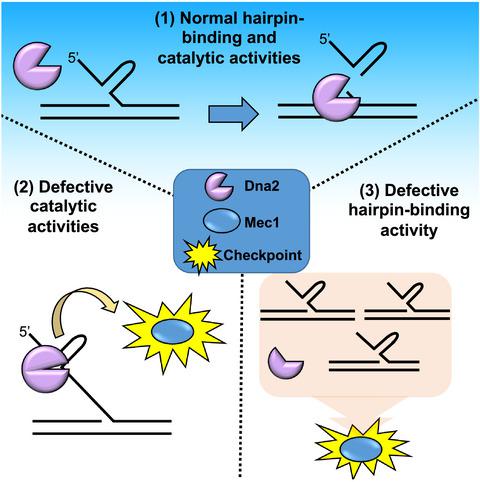
Dna2 can efficiently process 5′ flaps containing DNA secondary structure using coordinated action of the three biochemical activities: the N‐terminally encoded DNA‐binding activity and the C‐terminally encoded endonuclease and helicase activities. In this study, we investigated the cross talk among the three functional domains using a variety of dna2 mutant alleles and enzymes derived thereof. We found that disruption of the catalytic activities of Dna2 activated Dna2‐dependent checkpoint, residing in the N‐terminal domain. This checkpoint activity contributed to growth defects of dna2 catalytic mutants, revealing the presence of an intramolecular functional cross talk in Dna2. The N‐terminal domain of Dna2 bound specifically to substrates that mimic DNA replication fork intermediates, including Holliday junctions. Using site‐directed mutagenesis of the N‐terminal domain of Dna2, we discovered that five consecutive basic amino acid residues were essential for the ability of Dna2 to bind hairpin DNA in vitro. Mutant cells expressing the dna2 allele containing all five basic residues substituted with alanine displayed three distinct phenotypes: (i) temperature‐sensitive growth defects, (ii) bypass of S‐phase arrest, and (iii) increased sensitivity to DNA‐damaging agents. Taken together, our results indicate that the interplay between the N‐terminal regulatory and C‐terminal catalytic domains of Dna2 plays an important role in vivo, especially when cells are placed under replication stress.
中文翻译:

Dna2核酸内切酶/解旋酶的二级结构DNA结合活性对于复制压力下的细胞生长至关重要
Dna2可以通过三种生化活性的协同作用有效地处理含有DNA二级结构的5'襟翼:N端编码的DNA结合活性和C端编码的核酸内切酶和解旋酶活性。在这项研究中,我们使用各种dna2突变等位基因及其衍生的酶研究了三个功能域之间的串扰。我们发现破坏Dna2的催化活性激活了依赖于Dna2的检查点,该检查点位于N末端域。此检查点活动导致dna2的生长缺陷催化突变体,揭示了Dna2中分子内功能性串扰的存在。Dna2的N末端结构域与模拟DNA复制叉状中间体(包括霍利迪结)的底物特异性结合。使用Dna2 N末端结构域的定点诱变,我们发现五个连续的碱性氨基酸残基对于Dna2体外结合发夹DNA的能力至关重要。表达dna2的突变细胞包含全部五个被丙氨酸取代的基本残基的等位基因显示出三种不同的表型:(i)温度敏感的生长缺陷,(ii)绕过S期阻滞,以及(iii)对DNA破坏剂的敏感性增加。两者合计,我们的结果表明Dna2的N末端调节域和C末端催化域之间的相互作用在体内起着重要作用,尤其是当细胞处于复制压力下时。
更新日期:2020-07-07
中文翻译:

Dna2核酸内切酶/解旋酶的二级结构DNA结合活性对于复制压力下的细胞生长至关重要
Dna2可以通过三种生化活性的协同作用有效地处理含有DNA二级结构的5'襟翼:N端编码的DNA结合活性和C端编码的核酸内切酶和解旋酶活性。在这项研究中,我们使用各种dna2突变等位基因及其衍生的酶研究了三个功能域之间的串扰。我们发现破坏Dna2的催化活性激活了依赖于Dna2的检查点,该检查点位于N末端域。此检查点活动导致dna2的生长缺陷催化突变体,揭示了Dna2中分子内功能性串扰的存在。Dna2的N末端结构域与模拟DNA复制叉状中间体(包括霍利迪结)的底物特异性结合。使用Dna2 N末端结构域的定点诱变,我们发现五个连续的碱性氨基酸残基对于Dna2体外结合发夹DNA的能力至关重要。表达dna2的突变细胞包含全部五个被丙氨酸取代的基本残基的等位基因显示出三种不同的表型:(i)温度敏感的生长缺陷,(ii)绕过S期阻滞,以及(iii)对DNA破坏剂的敏感性增加。两者合计,我们的结果表明Dna2的N末端调节域和C末端催化域之间的相互作用在体内起着重要作用,尤其是当细胞处于复制压力下时。











































 京公网安备 11010802027423号
京公网安备 11010802027423号