当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-dimensional environment and vascularization induce osteogenic maturation of human adipose-derived stem cells comparable to that of bone-derived progenitors.
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-07-08 , DOI: 10.1002/sctm.19-0207 Amel Ibrahim 1, 2 , Naiara Rodriguez-Florez 1, 2, 3 , Oliver F W Gardner 1 , Eleonora Zucchelli 1 , Sophie E P New 1 , Alessandro Borghi 1, 2 , David Dunaway 1, 2 , Neil W Bulstrode 2 , Patrizia Ferretti 1
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-07-08 , DOI: 10.1002/sctm.19-0207 Amel Ibrahim 1, 2 , Naiara Rodriguez-Florez 1, 2, 3 , Oliver F W Gardner 1 , Eleonora Zucchelli 1 , Sophie E P New 1 , Alessandro Borghi 1, 2 , David Dunaway 1, 2 , Neil W Bulstrode 2 , Patrizia Ferretti 1
Affiliation
|
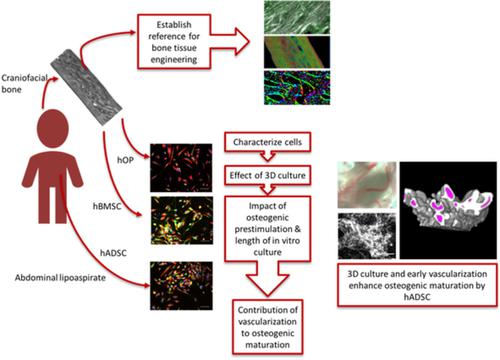
While human adipose‐derived stem cells (hADSCs) are known to possess osteogenic differentiation potential, the bone tissues formed are generally considered rudimentary and immature compared with those made by bone‐derived precursor cells such as human bone marrow‐derived mesenchymal stem cells (hBMSCs) and less commonly studied human calvarium osteoprogenitor cells (hOPs). Traditional differentiation protocols have tended to focus on osteoinduction of hADSCs through the addition of osteogenic differentiation media or use of stimulatory bioactive scaffolds which have not resulted in mature bone formation. Here, we tested the hypothesis that by reproducing the physical as well as biochemical bone microenvironment through the use of three‐dimensional (3D) culture and vascularization we could enhance osteogenic maturation in hADSCs. In addition to biomolecular characterization, we performed structural analysis through extracellular collagen alignment and mineral density in our bone tissue engineered samples to evaluate osteogenic maturation. We further compared bone formed by hADSCs, hBMSCs, and hOPs against mature human pediatric calvarial bone, yet not extensively investigated. Although bone generated by all three cell types was still less mature than native pediatric bone, a fibrin‐based 3D microenvironment together with vascularization boosted osteogenic maturation of hADSC making it similar to that of bone‐derived osteoprogenitors. This demonstrates the important role of vascularization and 3D culture in driving osteogenic maturation of cells easily available but constitutively less committed to this lineage and suggests a crucial avenue for recreating the bone microenvironment for tissue engineering of mature craniofacial bone tissues from pediatric hADSCs, as well as hBMSCs and hOPs.
中文翻译:

三维环境和血管化诱导人脂肪源性干细胞的成骨成熟,与骨源性祖细胞的成骨成熟相当。
虽然已知人脂肪源性干细胞 (hADSCs) 具有成骨分化潜能,但与由骨源性前体细胞如人骨髓间充质干细胞 (hBMSCs ) 和不太常研究的人颅骨骨祖细胞 (hOP)。传统的分化方案倾向于通过添加成骨分化培养基或使用未导致成熟骨形成的刺激性生物活性支架来对 hADSCs 进行骨诱导。在这里,我们测试了这样一个假设,即通过使用三维 (3D) 培养和血管化再现物理和生化骨微环境,我们可以增强 hADSCs 的成骨成熟。除了生物分子表征外,我们还通过骨组织工程样品中的细胞外胶原排列和矿物质密度进行结构分析,以评估成骨成熟。我们进一步将 hADSCs、hBMSCs 和 hOPs 形成的骨骼与成熟的人类儿科颅骨进行了比较,但尚未进行广泛研究。尽管所有三种细胞类型生成的骨骼仍然不如天然儿科骨骼成熟,但基于纤维蛋白的 3D 微环境和血管化促进了 hADSC 的成骨成熟,使其类似于骨源性骨祖细胞的成骨成熟。
更新日期:2020-07-08
中文翻译:

三维环境和血管化诱导人脂肪源性干细胞的成骨成熟,与骨源性祖细胞的成骨成熟相当。
虽然已知人脂肪源性干细胞 (hADSCs) 具有成骨分化潜能,但与由骨源性前体细胞如人骨髓间充质干细胞 (hBMSCs ) 和不太常研究的人颅骨骨祖细胞 (hOP)。传统的分化方案倾向于通过添加成骨分化培养基或使用未导致成熟骨形成的刺激性生物活性支架来对 hADSCs 进行骨诱导。在这里,我们测试了这样一个假设,即通过使用三维 (3D) 培养和血管化再现物理和生化骨微环境,我们可以增强 hADSCs 的成骨成熟。除了生物分子表征外,我们还通过骨组织工程样品中的细胞外胶原排列和矿物质密度进行结构分析,以评估成骨成熟。我们进一步将 hADSCs、hBMSCs 和 hOPs 形成的骨骼与成熟的人类儿科颅骨进行了比较,但尚未进行广泛研究。尽管所有三种细胞类型生成的骨骼仍然不如天然儿科骨骼成熟,但基于纤维蛋白的 3D 微环境和血管化促进了 hADSC 的成骨成熟,使其类似于骨源性骨祖细胞的成骨成熟。









































 京公网安备 11010802027423号
京公网安备 11010802027423号