Water Research ( IF 11.4 ) Pub Date : 2020-07-08 , DOI: 10.1016/j.watres.2020.116123 Jiwoon Ra 1 , Hoonsik Yoom 2 , Heejong Son 2 , Yunho Lee 1
|
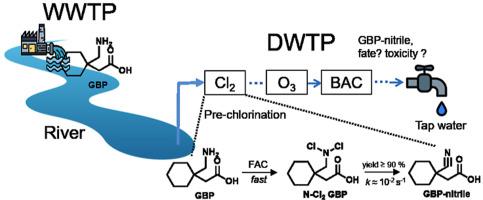
The occurrence and fate of the popular pharmaceutical gabapentin (GBP) in the urban water cycle were investigated with a focus on its transformation during water chlorination. GBP was detected in all samples with average concentrations of 1285 ng/L (n = 24) for wastewater effluent, 304 ng/L for river water (n = 22), and 180 ng/L for drinking water treatment plant (DWTP) influent (n = 4). The monitoring sites were located in the Nakdong River watershed, Korea. GBP was rapidly (within 20 min) transformed into 1-cyanocyclohexylacetic acid (GBP-nitrile) under typical chlorination conditions (1.4 mgCl2/L). When there was a molar excess of chlorine to GBP, the primary amine of GBP was double-chlorinated to form N-Cl2 GBP with a second-order rate constant of >103 M−1 s−1. Decomposition of N-Cl2 GBP had a first-order rate constant of (0.5–1.0) × 10−2 s−1 and produced GBP-nitrile with a yield of 87%–100%. We propose that N-Cl2 GBP is transformed into N-Cl GBP imine and then to GBP-nitrile via two consecutive dehydrochlorinations with the former as the rate-limiting step. N-Cl2 GBP had a much higher decomposition rate than N-Cl2 produced from other simple aliphatic amines, which could be related to the structural features of GBP such as its carboxyl group and quaternary β-carbon. The wastewater effluent samples did not contain GBP-nitrile even in the chlorinated effluent because of the relatively low chlorine dose or high ammonia level. In a full-scale DWTP employing a pre-chlorination unit, GBP present in the influent river water was fully transformed into GBP-nitrile. The formed GBP-nitrile was degraded in subsequent ozonation (•OH oxidation) and biological activated carbon filtration (biodegradation) processes. The toxicity of GBP-nitrile is thought to be low but further studies are warranted to assess the toxicological relevance of nitrile formation during water chlorination.
中文翻译:

加巴喷丁在城市水质工程中的发生和转化:饮用水加氯过程中胺快速生成腈。
研究了流行的加巴喷丁(GBP)在城市水循环中的发生和命运,重点是在水氯化过程中其转化。在所有样品中均检测到GBP ,废水流出物的平均浓度为1285 ng / L(n = 24),河水的平均浓度为304 ng / L(n = 22),饮用水处理厂(DWTP)的平均浓度为180 ng / L。 (n = 4)。监测地点位于韩国那洞河流域。在典型的氯化条件下(1.4 mgCl 2 / L),GBP迅速(在20分钟内)转化为1-氰基环己基乙酸(GBP-腈)。当氯对GBP的摩尔过量时,GBP的伯胺被双氯化以形成N-Cl 2GBP,其二阶速率常数> 10 3 M -1 s -1。N-Cl 2 GBP的分解具有(0.5–1.0)×10 -2 s -1的一级速率常数,并产生了GBP-腈,产率为87%–100%。我们建议将N-Cl 2 GBP转化为N-Cl GBP亚胺,然后通过两次连续的脱氯化氢反应将其转化为GBP-腈,前者作为限速步骤。N-Cl 2 GBP的分解速率比N-Cl 2高得多由其他简单的脂肪胺生成的,可能与GBP的结构特征(如羧基和季碳)有关。由于相对较低的氯剂量或较高的氨含量,即使在氯化的废水中,废水的废水样品也不包含GBP腈。在采用预氯化装置的大规模DWTP中,流入河水中的GBP完全转化为GBP腈。形成的GBP腈在随后的臭氧化(• OH氧化)和生物活性炭过滤(生物降解)过程中降解。人们认为,GBP腈的毒性较低,但是有必要进行进一步的研究以评估水氯化过程中腈形成的毒理学意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号