Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-07-08 , DOI: 10.1016/j.molliq.2020.113765 Yurong Zhao , Xingfan Li , Limin Zhang , Dong Wang , Wenxin Wang , Li Wang , Cuixia Chen
|
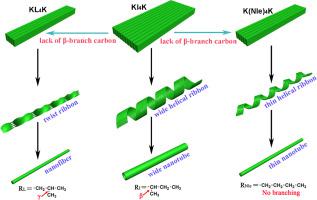
The structure of amino acids in peptides significantly affect the supramolecular architecture. Even small changes in the peptide molecular structure can have dramatic impact on the morphology of the assemblies. Herein, we demonstrate that the replacement of the four hydrophobic amino acid residues I in Ac-KI4K-NH2 (KI4K) for nleucine (Nle), leucine (L), and tert-leucine (Tle) can greatly affect the aggregate morphology and their self-assembling abilities. As for KI4K, the β-branch carbon in the hydrophobic amino acid I greatly promotes the lateral association of β-sheets and facilitate the formation of wider nanotubes. When the four hydrophobic residues I was replaced by Nle or L, thin nanotubes with quite small inner diameter were dominant for Ac-K(Nle)4K-NH2 (K(Nle)4K) while only thin nanofibers can be obtained for Ac-KL4K-NH2 (KL4K), suggesting the important roles of the four β-branch carbon in promoting the lateral association of β-sheets. On the contrary, no obvious aggregate structure was observed for Ac-K(Tle)4K-NH2 (K(Tle)4K) due to the strong steric hindrance interactions. Small angle neutron scattering (SANS) results indicated the different inner structure information for the nanotubes and nanofibers even though they share the same basic lamellar structure. Circular dichroism (CD), Fourier transform infrared spectroscopy (FTIR), and thioflavin-T (ThT) experiments indicated their different secondary structure and self-assembling abilities. These results can help us further understand the roles of β-branch carbon in controlling the aggregate morphology, which can benefit further research in establishing the relationship between the molecular structure and the final aggregate morphology.
中文翻译:

通过疏水氨基酸的侧链变异来调节超短波拉肽的自组装纳米结构
肽中氨基酸的结构会显着影响超分子结构。肽分子结构的即使很小的变化也会对组装体的形态产生巨大影响。在本文中,我们证明了Ac-KI 4 K-NH 2(KI 4 K)中的四个疏水氨基酸残基I替换为亮氨酸(Nle),亮氨酸(L)和叔亮氨酸(Tle)可能会产生很大影响聚集体形态及其自组装能力。至于KI 4疏水氨基酸I中的β-分支碳K极大地促进了β-折叠的侧向缔合并促进了更宽的纳米管的形成。当四个疏水残基I被Nle或L取代时,内径很小的细纳米管占主导地位的Ac-K(Nle)4 K-NH 2(K(Nle)4 K)而只有细纳米纤维可用于Ac-KL 4 K-NH 2(KL 4 K),表明四个β-分支碳在促进β-折叠的横向缔合中的重要作用。相反,对于Ac-K(Tle)4 K-NH 2(K(Tle)4K)由于强烈的位阻相互作用。小角度中子散射(SANS)结果表明,即使纳米管和纳米纤维具有相同的基本层状结构,它们的内部结构信息也不同。圆二色性(CD),傅立叶变换红外光谱(FTIR)和硫代黄素-T(ThT)实验表明它们的二级结构和自组装能力不同。这些结果可以帮助我们进一步了解β-分支碳在控制聚集体形态方面的作用,这有助于进一步研究建立分子结构与最终聚集体形态之间的关系。










































 京公网安备 11010802027423号
京公网安备 11010802027423号