Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-07-08 , DOI: 10.1016/j.yjmcc.2020.07.001 Lisa Hobuß 1 , Ariana Foinquinos 1 , Mira Jung 1 , Franziska Kenneweg 1 , Ke Xiao 1 , Yong Wang 2 , Karina Zimmer 1 , Janet Remke 1 , Annette Just 1 , Juliette Nowak 1 , Arne Schmidt 1 , Andreas Pich 3 , Stephane Mazlan 4 , Stella M Reamon-Buettner 5 , Gustavo Campos Ramos 6 , Stefan Frantz 6 , Janika Viereck 1 , Xavier Loyer 4 , Chantal Boulanger 4 , Kai C Wollert 2 , Jan Fiedler 1 , Thomas Thum 7
|
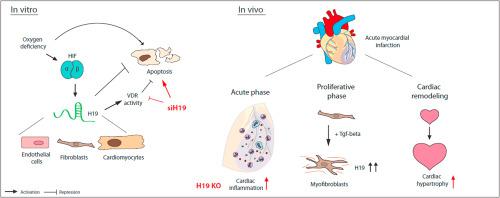
Myocardial ischemia induces a multifaceted remodeling process in the heart. Novel therapeutic entry points to counteract maladaptive signalling include the modulation of non-coding RNA molecules such as long non-coding RNA (lncRNA). We here questioned if the lncRNA candidate H19 exhibits regulatory potential in the setting of myocardial infarction. Initial profiling of H19 expression revealed a dynamic expression profile of H19 with upregulation in the acute phase after murine cardiac ischemia. In vitro, we found that oxygen deficiency leads to H19 upregulation in several cardiac cell types. Repression of endogenous H19 caused multiple phenotypes in cultivated murine cardiomyocytes including enhanced cardiomyocyte apoptosis, at least partly through attenuated vitamin D signalling. Unbiased proteome analysis revealed further involvement of H19 in mRNA splicing and translation as well as inflammatory signalling pathways. To study H19 function more precisely, we investigated the phenotype of systemic H19 loss in a genetic mouse model of H19 deletion (H19 KO). Infarcted heart tissue of H19 KO mice showed a massive increase of pro-inflammatory cytokines after ischemia-reperfusion injury (I/R) without significant effects on scar formation or cardiac function but exaggerated cardiac hypertrophy indicating pathological cardiac remodeling. H19-dependent changes in cardiomyocyte-derived extracellular vesicle release and alterations in NF-κB signalling were evident. Cardiac cell fractionation experiments revealed that enhanced H19 expression in the proliferative phase after MI derived mainly from cardiac fibroblasts. Here further research is needed to elucidate its role in fibroblast activation and function. In conclusion, the lncRNA H19 is dynamically regulated after MI and involved in multiple pathways of different cardiac cell types including cardiomyocyte apoptosis and cardiac inflammation.
中文翻译:

多发性心脏功能受缺血诱导的lncRNA H19控制。
心肌缺血在心脏中引起多方面的重塑过程。抵消适应不良信号的新治疗入口包括对非编码RNA分子(如长非编码RNA(lncRNA))的调节。我们在这里质疑lncRNA候选物H19在心肌梗塞的情况下是否表现出调节潜力。H19表达的初步分析揭示了小鼠心脏缺血后急性期中H19的动态表达特征。在体外,我们发现缺氧会导致几种心脏细胞类型的H19上调。抑制内源性H19导致至少部分通过减弱的维生素D信号传导导致培养的鼠心肌细胞出现多种表型,包括增强的心肌细胞凋亡。公正的蛋白质组分析显示H19进一步参与了mRNA的剪接和翻译以及炎症信号转导途径。为了更精确地研究H19功能,我们研究了H19缺失(H19 KO)的遗传小鼠模型中全身性H19丢失的表型。H19的梗死心脏组织KO小鼠在缺血再灌注损伤(I / R)后显示出促炎性细胞因子的大量增加,而对疤痕形成或心脏功能没有明显影响,但夸张的心脏肥大表明病理性心脏重塑。心肌细胞衍生的胞外小泡释放中H19依赖的变化和NF-κB信号的改变是明显的。心肌细胞分离实验显示,MI后增殖期的H19表达增强,其主要来源于心脏成纤维细胞。在这里,需要进一步的研究来阐明其在成纤维细胞活化和功能中的作用。总之,lncRNA H19 在心肌梗死后被动态调节,并参与不同心脏细胞类型的多种途径,包括心肌细胞凋亡和心脏炎症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号