European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-07-08 , DOI: 10.1016/j.ejmech.2020.112558 Cyril Fersing 1 , Clotilde Boudot 2 , Caroline Castera-Ducros 1 , Emilie Pinault 3 , Sébastien Hutter 4 , Romain Paoli-Lombardo 1 , Nicolas Primas 1 , Julien Pedron 5 , Line Seguy 5 , Sandra Bourgeade-Delmas 6 , Alix Sournia-Saquet 5 , Jean-Luc Stigliani 5 , Jean-Yves Brossas 7 , Luc Paris 7 , Alexis Valentin 6 , Susan Wyllie 8 , Alan H Fairlamb 8 , Élisa Boutet-Robinet 9 , Sophie Corvaisier 10 , Marc Since 10 , Aurélie Malzert-Fréon 10 , Alexandre Destere 11 , Dominique Mazier 12 , Pascal Rathelot 1 , Bertrand Courtioux 2 , Nadine Azas 4 , Pierre Verhaeghe 5 , Patrice Vanelle 1
|
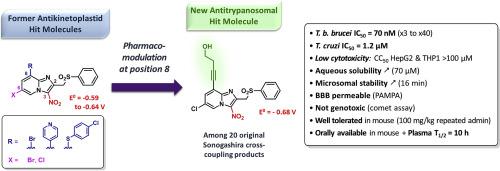
An antikinetoplastid pharmacomodulation study was done at position 8 of a previously identified pharmacophore in 3-nitroimidazo[1,2-a]pyridine series. Twenty original derivatives bearing an alkynyl moiety were synthesized via a Sonogashira cross-coupling reaction and tested in vitro, highlighting 3 potent (40 nM ≤ EC50 blood stream form≤ 70 nM) and selective (500 ≤ SI ≤ 1800) anti-T. brucei brucei molecules (19, 21 and 22), in comparison with four reference drugs. Among these hit molecules, compound 19 also showed the same level of activity against T. cruzi (EC50 amastigotes = 1.2 μM) as benznidazole and fexinidazole. An in vitro comet assay showed that nitroaromatic derivative 19 was not genotoxic. It displayed a low redox potential value (−0.68 V/NHE) and was shown to be bioactivated by type 1 nitroreductases both in Leishmania and Trypanosoma. The SAR study indicated that an alcohol function improved aqueous solubility while maintaining good activity and low cytotoxicity when the hydroxyl group was at position beta of the alkyne triple bond. Hit-compound 19 was also evaluated regarding in vitro pharmacokinetic data: 19 is BBB permeable (PAMPA assay), has a 16 min microsomal half-life and a high albumin binding (98.5%). Moreover, compound 19 was orally absorbed and was well tolerated in mouse after both single and repeated administrations at 100 mg/kg. Its mouse plasma half-life (10 h) is also quite encouraging, paving the way toward further efficacy evaluations in parasitized mouse models, looking for a novel antitrypanosomal lead compound.
中文翻译:

8-炔基-3-硝基咪唑并吡啶显示出对两种T都有力的抗锥虫活性。布鲁西和克鲁兹。
在3-硝基咪唑并[1,2- a ]吡啶系列中,在先前确定的药效基团的位置8进行了抗动素体药物代谢研究。通过Sonogashira交叉偶联反应合成了20个带有炔基的原始衍生物,并在体外进行了测试,突出了3种有效的(40 nM≤EC 50血流形式≤70 nM)和选择性(500≤SI≤1800)抗T.布氏锥虫分子(19,21和22),在具有四个参考药物比较。在这些命中分子中,化合物19也显示出相同水平的抗克鲁氏锥虫活性(EC 50amastigotes = 1.2μM)分别为苯硝唑和fexinidazole。的体外彗星试验表明,硝基芳族衍生物19是没有遗传毒性。它显示出较低的氧化还原电位值(-0.68 V / NHE),并且在利什曼原虫和锥虫中均被1型氮还原酶生物激活。SAR研究表明,当羟基位于炔烃三键的β位时,醇功能可改善水溶性,同时保持良好的活性和低细胞毒性。还针对体外药代动力学数据对命中化合物19进行了评估:19具有BBB渗透性(PAMPA测定),微粒体半衰期为16分钟,白蛋白结合率高(98.5%)。此外,在以100mg / kg单次和重复施用后,化合物19被口服吸收并且在小鼠中被良好耐受。它的小鼠血浆半衰期(10小时)也非常令人鼓舞,为进一步寻找寄生虫小鼠模型中的功效铺平了道路,以寻找新型的抗胰锥虫先导化合物。










































 京公网安备 11010802027423号
京公网安备 11010802027423号