Chemosphere ( IF 8.1 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.chemosphere.2020.127560 Elham Cheraghipour 1 , Mahmoud Pakshir 1
|
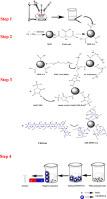
This study aimed to investigate the performance of a magnetic nano-biocomposite, chitosan conjugated magnetite nanoparticle (CH-MNP), for the removal of lead ions. The magnetite nanoparticles were synthesized through a controlled co-precipitation technique and were stabilized with citric acid. Subsequently, they were covalently bonded to chitosan via carbodiimide chemistry using EDAC/NHS activation. One of the notable advantages of this nano-biocomposite is its chemical conjugation, which does not have the weakness of the ultimate chitosan detachment of a physical bond and makes it an encouraging candidate for magnetic separation with no secondary waste production. The CH-MNPs had a diameter of ∼10 nm, with a saturation magnetization of 76.01 emu/g ensuring a superparamagnetic property. The response surface methodology (RSM) with a central composite design (CCD) framework was used for optimizing the adsorption process. The optimum conditions to achieve 92.15% of Pb(II) removal were found to be at a pH of 6.1 with the nano-adsorbent concentration of 1.04 g/L and a contact time of 59.92 min. Our adsorption isotherm data were fitted well with the Langmuir adsorption isotherm model, and the equilibrium data followed the pseudo-second-order kinetics and intraparticle diffusion kinetic model. The maximum Langmuir Pb(II) adsorption capacity was calculated to be 192.308 mg/g. These results suggest that the proposed synthetic nano-biocomposite is quite an ideal nano-adsorbent for Pb(II) removal in wastewater treatment technology.
中文翻译:

使用响应表面方法对壳聚糖-共轭磁铁矿纳米生物复合材料吸附Pb(II)离子的过程进行优化和建模。
这项研究旨在研究磁性纳米生物复合物,壳聚糖共轭磁铁矿纳米颗粒(CH-MNP)去除铅离子的性能。磁铁矿纳米粒子是通过控制共沉淀技术合成的,并用柠檬酸稳定。随后,使用EDAC / NHS活化通过碳二亚胺化学将它们共价键合至壳聚糖。这种纳米生物复合材料的显着优势之一是其化学共轭,它不具有最终的脱乙酰壳多糖物理键分离的弱点,并且使其成为磁分离的令人鼓舞的候选者,而不会产生二次废物。CH-MNP的直径约为10 nm,饱和磁化强度为76.01 emu / g,可确保超顺磁性。使用具有中央复合设计(CCD)框架的响应表面方法(RSM)来优化吸附过程。发现达到92.15%的Pb(II)去除的最佳条件是pH值为6.1,纳米吸附剂的浓度为1.04 g / L,接触时间为59.92分钟。我们的吸附等温线数据与Langmuir吸附等温线模型非常吻合,平衡数据遵循伪二级动力学和粒子内扩散动力学模型。计算得出的最大Langmuir Pb(II)吸附容量为192.308 mg / g。这些结果表明,所提出的合成纳米生物复合材料是用于废水处理技术中去除Pb(II)的理想纳米吸附剂。发现15%的Pb(II)的pH值为6.1,纳米吸附剂的浓度为1.04 g / L,接触时间为59.92分钟。我们的吸附等温线数据与Langmuir吸附等温线模型非常吻合,平衡数据遵循伪二级动力学和粒子内扩散动力学模型。计算得出的最大Langmuir Pb(II)吸附容量为192.308 mg / g。这些结果表明,所提出的合成纳米生物复合物是用于废水处理技术中去除Pb(II)的理想纳米吸附剂。发现15%的Pb(II)的pH值为6.1,纳米吸附剂的浓度为1.04 g / L,接触时间为59.92分钟。我们的吸附等温线数据与Langmuir吸附等温线模型非常吻合,平衡数据遵循伪二级动力学和粒子内扩散动力学模型。计算出的最大Langmuir Pb(II)吸附容量为192.308 mg / g。这些结果表明,所提出的合成纳米生物复合物是用于废水处理技术中去除Pb(II)的理想纳米吸附剂。平衡数据遵循拟二级动力学和粒子内扩散动力学模型。计算得出的最大Langmuir Pb(II)吸附容量为192.308 mg / g。这些结果表明,所提出的合成纳米生物复合物是用于废水处理技术中去除Pb(II)的理想纳米吸附剂。平衡数据遵循拟二级动力学和粒子内扩散动力学模型。计算得出的最大Langmuir Pb(II)吸附容量为192.308 mg / g。这些结果表明,所提出的合成纳米生物复合物是用于废水处理技术中去除Pb(II)的理想纳米吸附剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号