Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2020-07-08 , DOI: 10.1016/j.bbapap.2020.140485 Amrita Dawn 1 , Komal S Khatri 1 , Sandip Karmakar 1 , Shashank Deep 1
|
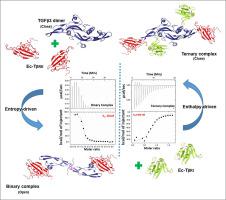
The proper orchestration of transforming growth factor beta (TGFβ) mediated signal transduction depends upon a delicate set of interactions between specific ligands and their receptors. Here we present an in-depth profiling of the binding mechanism of TGFβ3 ligand with its type II and type I receptors (TβRII and TβRI) using isothermal titration calorimetry (ITC). Studies were carried out in acidic pH as it has great physiological relevance for TGFβ3 activity. Our findings reveal an unusual positive enthalpy (∆H) compensated by a large favourable entropy (∆S) during TGFβ3-TβRII interaction. In addition to the hydrophobic effect, we propose that a distinct conformational switch from “closed” to “open” form as experienced by TGFβ3 on binding to TβRII is contributing significantly to the increase in overall entropy of the system. Binding studies of TGFβ3 and TβRII were carried out at different pH values and salt concentrations to gain further insight into the thermodynamics of the interaction. Furthermore, the importance of hydrophobic interactions on the binding affinity of TβRII with TGFβ3 was confirmed by two TβRII variants (interfacial). Finally, a distinct shift from entropy to enthalpy dominated interaction was observed upon recruitment of TβRI to the binary complex forming the ternary complex.
中文翻译:

TGFβ3配体与其II型受体(TβRII)和I型受体(TβRI)的相互作用:蛋白质-蛋白质缔合的独特机制。
转化生长因子β(TGFβ)介导的信号转导的适当编排取决于特定配体及其受体之间的微妙相互作用。在这里,我们介绍了使用等温滴定量热法(ITC)对TGFβ3配体与其II型和I型受体(TβRII和TβRI)的结合机理进行深入分析。由于它与TGFβ3活性具有很大的生理相关性,因此在酸性pH中进行了研究。我们的发现表明,在TGFβ3-TβRII相互作用期间,大的有利熵(ΔS)补偿了异常的正焓(ΔH)。除疏水作用外,我们认为TGFβ3与TβRII结合时经历了从“闭合”到“开放”形式的独特构象转换,这极大地促进了系统总体熵的增加。在不同的pH值和盐浓度下进行了TGFβ3和TβRII的结合研究,以进一步了解相互作用的热力学。此外,通过两个TβRII变体(界面)证实了疏水相互作用对TβRII与TGFβ3的结合亲和力的重要性。最后,在将TβRI募集至形成三元复合物的二元复合物时,观察到了从熵到焓主导相互作用的明显转变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号