当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly multiplexed quantitative proteomic and phosphoproteomic analyses in vascular smooth muscle cell dedifferentiation
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.aca.2020.06.054 Xiaofang Zhong 1 , Christopher B Lietz 2 , Xudong Shi 3 , Amanda R Buchberger 2 , Dustin C Frost 1 , Lingjun Li 4
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.aca.2020.06.054 Xiaofang Zhong 1 , Christopher B Lietz 2 , Xudong Shi 3 , Amanda R Buchberger 2 , Dustin C Frost 1 , Lingjun Li 4
Affiliation
|
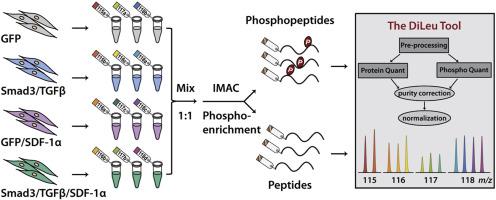
Restenosis, re-narrowing of arterial lumen following intervention for cardiovascular disease, remains a major issue limiting the long-term therapeutic efficacy of treatment. The signaling molecules, TGFβ (transforming growth factor-beta) and Smad3, play important roles in vascular restenosis, but very little is yet known about the down-stream dynamics in global protein expression and phosphorylation. Here, we develop a highly multiplexed quantitative proteomic and phosphoproteomic strategy employing 12-plex N,N-dimethyl leucine (DiLeu) isobaric tags and The DiLeu Tool software to globally assess protein expression and phosphorylation changes in smooth muscle cells (SMCs) treated with TGFβ/Smad3 and/or SDF-1α (stromal cell-derived factor). A total of 4086 proteins were quantified in the combined dataset of proteome and phosphoproteome across 12-plex DiLeu-labeled SMC samples. 2317 localized phosphorylation sites were quantified, corresponding to 1193 phosphoproteins. TGFβ/Smad3 induced up-regulation of 40 phosphosites and down-regulation of 50 phosphosites, and TGFβ/Smad3-specific SDF-1α exclusively facilitated up-regulation of 27 phosphosites and down-regulation of 47 phosphosites. TGFβ/Smad3 inhibited the expression of contractile-associated proteins including smooth muscle myosin heavy chain, calponin, cardiac muscle alpha-actin, and smooth muscle protein 22α. Gene ontology and pathway enrichment analysis revealed that elevated TGFβ/Smad3 activated cell proliferation and TGFβ signaling pathway, sequentially stimulating phosphorylation of CXCR4 (C-X-C chemokine receptor 4). SDF-1α/CXCR4 activated extracellular signal-regulating kinase signaling pathway and facilitated the expression of synthetic marker, osteopontin, which was validated through targeted analysis. These findings provide new insights into the mechanisms of TGFβ regulated SMC dedifferentiation, as well as new avenues for designing effective therapeutics for vascular disease.
中文翻译:

血管平滑肌细胞去分化中的高度多重定量蛋白质组学和磷酸蛋白质组学分析
心血管疾病干预后动脉管腔再狭窄,仍然是限制长期治疗效果的主要问题。信号分子 TGFβ(转化生长因子-β)和 Smad3 在血管再狭窄中起重要作用,但对全局蛋白质表达和磷酸化的下游动力学知之甚少。在这里,我们开发了一种高度多重的定量蛋白质组学和磷酸蛋白质组学策略,采用 12 重 N,N-二甲基亮氨酸 (DiLeu) 同量异位标记和 DiLeu 工具软件来全面评估用 TGFβ 处理的平滑肌细胞 (SMC) 中的蛋白质表达和磷酸化变化/Smad3 和/或 SDF-1α(基质细胞衍生因子)。在 12 重 DiLeu 标记的 SMC 样本的蛋白质组和磷酸蛋白质组的组合数据集中,总共量化了 4086 种蛋白质。量化了 2317 个局部磷酸化位点,对应于 1193 个磷蛋白。TGFβ/Smad3 诱导了 40 个磷酸位点的上调和 50 个磷酸位点的下调,并且 TGFβ/Smad3 特异性 SDF-1α 专门促进了 27 个磷酸位点的上调和 47 个磷酸位点的下调。TGFβ/Smad3 抑制收缩相关蛋白的表达,包括平滑肌肌球蛋白重链、钙调蛋白、心肌α-肌动蛋白和平滑肌蛋白 22α。基因本体和通路富集分析显示,升高的 TGFβ/Smad3 激活细胞增殖和 TGFβ 信号通路,依次刺激 CXCR4(CXC 趋化因子受体 4)的磷酸化。SDF-1α/CXCR4 激活细胞外信号调节激酶信号通路并促进合成标志物骨桥蛋白的表达,这通过靶向分析得到验证。这些发现提供了对 TGFβ 调节的 SMC 去分化机制的新见解,以及设计有效治疗血管疾病的新途径。
更新日期:2020-08-01
中文翻译:

血管平滑肌细胞去分化中的高度多重定量蛋白质组学和磷酸蛋白质组学分析
心血管疾病干预后动脉管腔再狭窄,仍然是限制长期治疗效果的主要问题。信号分子 TGFβ(转化生长因子-β)和 Smad3 在血管再狭窄中起重要作用,但对全局蛋白质表达和磷酸化的下游动力学知之甚少。在这里,我们开发了一种高度多重的定量蛋白质组学和磷酸蛋白质组学策略,采用 12 重 N,N-二甲基亮氨酸 (DiLeu) 同量异位标记和 DiLeu 工具软件来全面评估用 TGFβ 处理的平滑肌细胞 (SMC) 中的蛋白质表达和磷酸化变化/Smad3 和/或 SDF-1α(基质细胞衍生因子)。在 12 重 DiLeu 标记的 SMC 样本的蛋白质组和磷酸蛋白质组的组合数据集中,总共量化了 4086 种蛋白质。量化了 2317 个局部磷酸化位点,对应于 1193 个磷蛋白。TGFβ/Smad3 诱导了 40 个磷酸位点的上调和 50 个磷酸位点的下调,并且 TGFβ/Smad3 特异性 SDF-1α 专门促进了 27 个磷酸位点的上调和 47 个磷酸位点的下调。TGFβ/Smad3 抑制收缩相关蛋白的表达,包括平滑肌肌球蛋白重链、钙调蛋白、心肌α-肌动蛋白和平滑肌蛋白 22α。基因本体和通路富集分析显示,升高的 TGFβ/Smad3 激活细胞增殖和 TGFβ 信号通路,依次刺激 CXCR4(CXC 趋化因子受体 4)的磷酸化。SDF-1α/CXCR4 激活细胞外信号调节激酶信号通路并促进合成标志物骨桥蛋白的表达,这通过靶向分析得到验证。这些发现提供了对 TGFβ 调节的 SMC 去分化机制的新见解,以及设计有效治疗血管疾病的新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号