Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-07-08 , DOI: 10.1016/j.actbio.2020.07.009 Ana M Matos 1 , Ana I Gonçalves 1 , Márcia T Rodrigues 1 , Margarida S Miranda 1 , Alicia J El Haj 2 , Rui L Reis 1 , Manuela E Gomes 1
|
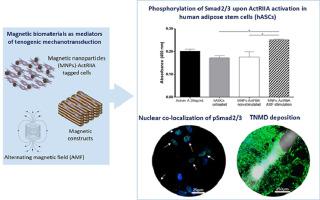
Injuries affecting load bearing tendon tissues are a significant clinical burden and efficient treatments are still unmet. Tackling tendon regeneration, tissue engineering strategies aim to develop functional substitutes that recreate native tendon milieu. Tendon mimetic scaffolds capable of remote magnetic responsiveness and functionalized magnetic nanoparticles (MNPs) targeting cellular mechanosensitive receptors are potential instructive tools to mediate mechanotransduction in guiding tenogenic responses. In this work, we combine magnetically responsive scaffolds and targeted Activin A type II receptor in human adipose stem cells (hASCs), under alternating magnetic field (AMF), to synergistically facilitate external control over signal transduction. The combination of remote triggering TGF-β/Smad2/3 using MNPs tagged hASCs, through magnetically actuated scaffolds, stimulates overall expression of tendon related genes and the deposition of tendon related proteins, in comparison to non-stimulated conditions. Moreover, the phosphorylation of Smad2/3 proteins and their nuclear co-localization was also more evident. Overall, biophysical stimuli resulting from magnetic scaffolds and magnetically triggered cells under AMF stimulation modulate the mechanosensing response of hASCs towards tenogenesis, holding therapeutic promise.
Statement of Significance
The concept of magnetically-assisted tissue engineering may assist the development of innovative solutions to treat tendon disorders upon remote control of biological processes as cell migration or differentiation. Herein, we originally combine a fibrous aligned superparamagnetic scaffold, based on a biodegradable polymeric blend of starch and poly-ɛ-caprolactone incorporating magnetic nanoparticles (MNPs), and human adipose stem cells (hASCs) labelled with MNPs functionalized with anti-activin receptor type IIA (ActRIIA). Constructs were stimulated using alternating magnetic field (AMF), to activate the ActRIIA and subsequent induction of TGF-β signaling, through Smad2/3 phosphorylation cascade, enhancing the expression of tendon-related markers. Altogether, these findings contribute with powerful bio-magnetic approaches to activate key tenogenic pathways, envisioning future translation of magnetic biomaterials into regenerative platforms for tendon repair.
中文翻译:

载于磁性支架上的人类脂肪干细胞中TGF-β/ Smad2 / 3信号的远程触发可协同促进肌腱形成。
影响承重肌腱组织的损伤是巨大的临床负担,有效的治疗仍未得到满足。解决肌腱再生问题,组织工程策略旨在开发可重建天然肌腱环境的功能替代品。能够实现远程磁响应和功能性磁性纳米粒子(MNP)靶向细胞机械敏感受体的腱模拟支架是潜在的指导工具,可在指导肌腱反应中介导机械转导。在这项工作中,我们将磁场响应支架与人类脂肪干细胞(hASCs)中的靶向激活素II型受体结合,并在交变磁场(AMF)的作用下,协同促进信号转导的外部控制。使用标记有hASC的MNP远程触发TGF-β/ Smad2 / 3的组合,与非刺激条件相比,通过磁力驱动的支架可以刺激肌腱相关基因的整体表达和肌腱相关蛋白的沉积。此外,Smad2 / 3蛋白的磷酸化及其核共定位也更加明显。总体而言,在AMF刺激下,由磁性支架和磁性触发的细胞产生的生物物理刺激可调节hASC对肌腱形成的机械传感反应,具有治疗前景。
重要声明
磁辅助组织工程学的概念可以协助开发创新解决方案,以远程控制生物过程(如细胞迁移或分化)来治疗肌腱疾病。在这里,我们最初结合了纤维排列的超顺磁性支架,该支架基于淀粉和聚ɛ-己内酯的可生物降解聚合物混合物,并掺入了磁性纳米颗粒(MNP)和标记有抗激活素受体类型功能化的MNP的人脂肪干细胞(hASC) IIA(ActRIIA)。使用交变磁场(AMF)刺激构建体,以激活ActRIIA并随后通过Smad2 / 3磷酸化级联反应诱导TGF-β信号传导,从而增强腱相关标志物的表达。共,











































 京公网安备 11010802027423号
京公网安备 11010802027423号