Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Real-world evaluation of a novel technology for quantitative simultaneous antibody detection against multiple SARS-CoV-2 antigens in a cohort of patients presenting with COVID-19 syndrome.
Analyst ( IF 3.6 ) Pub Date : 2020-07-07 , DOI: 10.1039/d0an01066a Andrew M Shaw 1 , Christopher Hyde 2 , Blair Merrick 3 , Philip James-Pemberton 1 , Bethany K Squires 4 , Rouslan V Olkhov 1 , Rahul Batra 3 , Amita Patel 3 , Karen Bisnauthsing 3 , Gaia Nebbia 3 , Eithne MacMahon 3 , Sam Douthwaite 5 , Michael Malim 5 , Stuart Neil 5 , Rocio Martinez Nunez 5 , Katie Doores 5 , Tan Kia Ik Mark 5 , Adrian W Signell 5 , Gilberto Betancor 5 , Harry D Wilson 5 , Rui Pedro Galão 5 , Suzanne Pickering 5 , Jonathan D Edgeworth 6
Analyst ( IF 3.6 ) Pub Date : 2020-07-07 , DOI: 10.1039/d0an01066a Andrew M Shaw 1 , Christopher Hyde 2 , Blair Merrick 3 , Philip James-Pemberton 1 , Bethany K Squires 4 , Rouslan V Olkhov 1 , Rahul Batra 3 , Amita Patel 3 , Karen Bisnauthsing 3 , Gaia Nebbia 3 , Eithne MacMahon 3 , Sam Douthwaite 5 , Michael Malim 5 , Stuart Neil 5 , Rocio Martinez Nunez 5 , Katie Doores 5 , Tan Kia Ik Mark 5 , Adrian W Signell 5 , Gilberto Betancor 5 , Harry D Wilson 5 , Rui Pedro Galão 5 , Suzanne Pickering 5 , Jonathan D Edgeworth 6
Affiliation
|
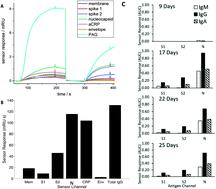
An evaluation of a rapid portable gold-nanotechnology measuring SARS-CoV-2 IgM, IgA and IgG antibody concentrations against spike 1 (S1), spike 2 (S) and nucleocapsid (N) was conducted using serum samples from 74 patients tested for SARS-CoV-2 RNA on admission to hospital, and 47 historical control patients from March 2019. 59 patients were RNA(+) and 15 were RNA(−). A serum (±) classification was derived for all three antigens and a quantitative serological profile was obtained. Serum(+) was identified in 30% (95% CI 11–48) of initially RNA(−) patients, in 36% (95% CI 17–54) of RNA(+) patients before 10 days, 77% (95% CI 67–87) between 10 and 20 days and 95% (95% CI 86–100) after 21 days. The patient-level diagnostic accuracy relative to RNA(±) after 10 days displayed 88% sensitivity (95% CI 75–95) and 75% specificity (95% CI 22–99), although specificity compared with historical controls was 100% (95%CI 91–100). This study provides robust support for further evaluation and validation of this novel technology in a clinical setting and highlights challenges inherent in assessment of serological tests for an emerging disease such as COVID-19.
中文翻译:

对一种新技术的真实世界评估,该技术可在一组患有 COVID-19 综合征的患者中同时定量检测针对多种 SARS-CoV-2 抗原的抗体。
使用 74 名接受 SARS 检测的患者的血清样本,对快速便携式金纳米技术测量针对尖峰 1 (S1)、尖峰 2 (S) 和核衣壳 (N) 的 SARS-CoV-2 IgM、IgA 和 IgG 抗体浓度进行了评估-入院时的 CoV-2 RNA,以及 2019 年 3 月以来的 47 名历史对照患者。59 名患者为 RNA(+),15 名患者为 RNA(−)。对所有三种抗原进行血清 (±) 分类,并获得定量血清学谱。 30% (95% CI 11–48) 的最初 RNA(−) 患者中检测到血清(+),36% (95% CI 17–54) 的 RNA(+) 患者在 10 天前检测到血清(+),77% (95 10 至 20 天之间为 % CI 67–87),21 天后为 95% (95% CI 86–100)。 10 天后相对于 RNA(±) 的患者水平诊断准确性显示出 88% 的敏感性 (95% CI 75-95) 和 75% 的特异性 (95% CI 22-99),尽管与历史对照相比特异性为 100%( 95% CI 91–100)。这项研究为在临床环境中进一步评估和验证这项新技术提供了强有力的支持,并强调了评估新发疾病(如 COVID-19)的血清学检测所固有的挑战。
更新日期:2020-08-10
中文翻译:

对一种新技术的真实世界评估,该技术可在一组患有 COVID-19 综合征的患者中同时定量检测针对多种 SARS-CoV-2 抗原的抗体。
使用 74 名接受 SARS 检测的患者的血清样本,对快速便携式金纳米技术测量针对尖峰 1 (S1)、尖峰 2 (S) 和核衣壳 (N) 的 SARS-CoV-2 IgM、IgA 和 IgG 抗体浓度进行了评估-入院时的 CoV-2 RNA,以及 2019 年 3 月以来的 47 名历史对照患者。59 名患者为 RNA(+),15 名患者为 RNA(−)。对所有三种抗原进行血清 (±) 分类,并获得定量血清学谱。 30% (95% CI 11–48) 的最初 RNA(−) 患者中检测到血清(+),36% (95% CI 17–54) 的 RNA(+) 患者在 10 天前检测到血清(+),77% (95 10 至 20 天之间为 % CI 67–87),21 天后为 95% (95% CI 86–100)。 10 天后相对于 RNA(±) 的患者水平诊断准确性显示出 88% 的敏感性 (95% CI 75-95) 和 75% 的特异性 (95% CI 22-99),尽管与历史对照相比特异性为 100%( 95% CI 91–100)。这项研究为在临床环境中进一步评估和验证这项新技术提供了强有力的支持,并强调了评估新发疾病(如 COVID-19)的血清学检测所固有的挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号