Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-05-31 , DOI: 10.2174/1570180816666190913183623 Pulabala Ramesh 1 , Vankadari Srinivasa Rao 1 , Puchakayala Muralidhar Reddy 1 , Katragadda Suresh Babu 2 , Mutheneni Srinivasa Rao 3
|
Background: Most of the currently available pharmaceutical drugs are either natural products or analogues of natural products. Flavonoids are plant based natural polyphenolic compounds which exhibit a wide range of biological activities. Chrysin, a natural flavone, exhibits several biological activities like antiallergic, anti-inflammatory and anticancer. Many efforts were made to enhance the biological activity of chrysin. In continuation of our work on synthetic modifications of chrysin, amino-alcohol containing heterocyclic moiety is linked to chrysin at C (7) position to enhance its biological activity.
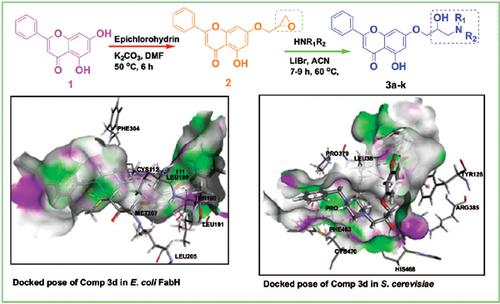
Methods: A series of new C (7) modified analogues of chrysin (3a-k) have been designed and synthesized in two steps. Chrysin, on reacting with epichlorohydrin in the presence of K2CO3 in DMF gave epoxide (2) which was made to react with cyclic secondary amines in the presence of LiBr to form the designed products (3a-k). All the synthesized compounds (3a-k) were well characterized by 1H NMR, 13C NMR and mass spectral data. The synthesized analogues (3a-k) were screened for their in vitro biological activities against a panel of bacterial and fungal strains. Molecular docking studies were also performed on these compounds with E. coli FabH (1HNJ) and S. cerevisiae (5EQB) enzymes, to support the observed biological activities.
Results: A series of new 2-hydroxy 3-amino chrysin derivatives (3a-k) were synthesized in two steps, starting with chrysin and their structures were characterized by spectral analysis. In vitro biological activities of these analogues against a panel of bacterial and fungal strains indicated that some of the derivatives manifested significant activities compared to standard drugs. Molecular docking and binding energy values were also correlated with experimental antimicrobial screening results. Lipinski’s “rule of five” is also obeyed by these analogues (3a-k) and exhibit drug-likeness.
Conclusion: In the present study, a series of new C (7) modified chrysin analogues (3a-k) were synthesized and tested for their in vitro antimicrobial activities. These biological studies indicated that some of the derivatives exhibited moderate to good antimicrobial activities compared to standard drugs. Molecular docking studies performed on these compounds correlated with the experimental antimicrobial activities. The results obtained in the study will be useful in establishing new drug entities to control the pathogenic epidemics.
中文翻译:

新的C(7)修饰的类似物的合成,生物学评价和分子建模研究。
背景:目前可用的大多数药物是天然产物或天然产物的类似物。类黄酮是基于植物的天然多酚化合物,具有广泛的生物活性。天然黄酮菊花素具有多种生物活性,如抗过敏,抗炎和抗癌作用。进行了许多努力来增强菊花素的生物活性。在继续进行关于菊花素的合成修饰的工作中,含氨基醇的杂环部分在C(7)位置与菊花素相连,以增强其生物学活性。
方法:设计并合成了一系列新的C(7)修饰的菊花(3a-k)类似物,并分两步合成。在DMF中,在K 2 CO 3存在下,Chrysin与表氯醇反应,得到环氧化物(2),使其在LiBr存在下与环状仲胺反应形成设计的产物(3a-k)。通过1 H NMR,13 C NMR和质谱数据对所有合成的化合物(3a-k)进行了很好的表征。筛选合成的类似物(3a-k)对一组细菌和真菌菌株的体外生物活性。还使用大肠杆菌FabH(1HNJ)和啤酒酵母(5EQB)酶对这些化合物进行了分子对接研究,以支持所观察到的生物学活性。
结果:从白蛋白开始,分两步合成了一系列新的2-羟基3-氨基白蛋白衍生物(3a-k),并通过光谱分析对其结构进行了表征。这些类似物对一组细菌和真菌菌株的体外生物学活性表明,与标准药物相比,某些衍生物表现出显着的活性。分子对接和结合能值也与实验性抗菌筛选结果相关。这些类似物(3a-k)也遵守Lipinski的“五个规则”,并表现出类似药物的作用。
结论:在本研究中,合成了一系列新的C(7)修饰的菊花素类似物(3a-k),并测试了它们的体外抗菌活性。这些生物学研究表明,与标准药物相比,某些衍生物具有中等至良好的抗菌活性。对这些化合物进行的分子对接研究与实验性抗菌活性相关。研究中获得的结果将有助于建立新的药物实体来控制病原性流行病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号