当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Total Synthesis of Bufospirostenin A
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-06 , DOI: 10.1021/jacs.0c05479 Min-Jing Cheng 1, 2 , Li-Ping Zhong 2 , Chen-Chen Gu 2 , Xu-Jiang Zhu 2 , Bo Chen 2 , Jun-Shan Liu 3 , Lei Wang 1 , Wen-Cai Ye 1 , Chuang-Chuang Li 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-06 , DOI: 10.1021/jacs.0c05479 Min-Jing Cheng 1, 2 , Li-Ping Zhong 2 , Chen-Chen Gu 2 , Xu-Jiang Zhu 2 , Bo Chen 2 , Jun-Shan Liu 3 , Lei Wang 1 , Wen-Cai Ye 1 , Chuang-Chuang Li 2
Affiliation
|
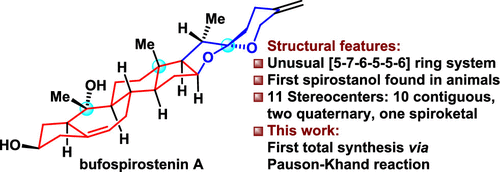
The first and asymmetric total synthesis of bioactive bufospirostenin A, an unusual spirostanol with rearranged A/B rings, was accomplished. The synthetically challenging [5-7-6-5] tetracyclic ring system, found in bufospirostenin A and some other natural products, was efficiently constructed by the unique intramolecular rhodium-catalyzed Pauson-Khand reaction of an alkoxyallene-yne. The 11 stereocenters in the final product, including the 10 contiguous stereocenters, were installed diastereoselectively.
中文翻译:

Bufospirostenin A的不对称全合成
首次和不对称全合成生物活性 bufospirostenin A,一种具有重排 A/B 环的不寻常螺甾醇,已完成。在 bufospirostenin A 和一些其他天然产物中发现的合成具有挑战性的 [5-7-6-5] 四环系统是通过独特的分子内铑催化的烷氧基丙二烯-炔的 Pauson-Khand 反应有效构建的。最终产品中的 11 个立体中心,包括 10 个连续的立体中心,是非对映选择性安装的。
更新日期:2020-07-06
中文翻译:

Bufospirostenin A的不对称全合成
首次和不对称全合成生物活性 bufospirostenin A,一种具有重排 A/B 环的不寻常螺甾醇,已完成。在 bufospirostenin A 和一些其他天然产物中发现的合成具有挑战性的 [5-7-6-5] 四环系统是通过独特的分子内铑催化的烷氧基丙二烯-炔的 Pauson-Khand 反应有效构建的。最终产品中的 11 个立体中心,包括 10 个连续的立体中心,是非对映选择性安装的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号