当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Determination and Modeling for Tirofiban in Several Mixed Solvents at 278.15–323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.jced.0c00371 Jianqiang Zhang 1 , Haixia Zhang 2 , Renjie Xu 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.jced.0c00371 Jianqiang Zhang 1 , Haixia Zhang 2 , Renjie Xu 2
Affiliation
|
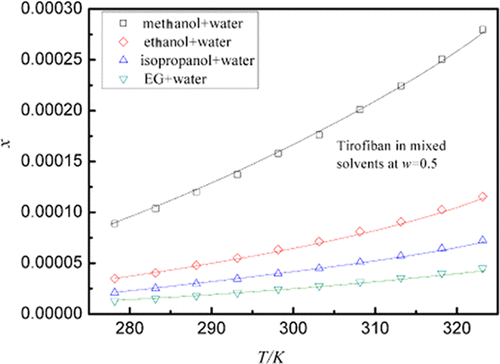
During the determination of the solubility of tirofiban, pure methanol, ethanol, isopropanol, and ethylene glycol (EG) play a major role in dissolution; then, water is added in sequence for dilution to obtain a dispersion system with different composition structures, and the concentration gradient between each mixed liquid is 0.1 (mass fraction). The drug tirofiban whose solubility is to be tested is added to it, using the isothermal method, with a high temperature of 323.15 K as the starting point and an interval of 5 K to a low temperature of 278.15 K. The pressure conditions do not require special requirements at ordinary atmospheric pressure environment (101.1 kPa). Results show that when the temperature approaches the high level, more drugs can be dissolved in the same amount of solvent. Conversely, when the temperature is close to the low level, the drug is more difficult to dissolve. The temperature constant, the proportion of organic solvent is large, the dissolving capacity of the dispersion system will develop in a positive direction. Conversely, if the proportion of water is large, the dissolving power will decrease. Tirofiban crystals accepted the determination of various dissolution data, including those of the undissolved raw materials that have undergone X-ray power diffraction (XPRD) detection. The results obtained are encouraging, no change in the crystal structure of the substance is found. Three models, Jouyban–Acree model, van’t Hoff–Jouyban–Acree model, and modified Apelblat–Jouyban–Acree model, were employed to calculate the experimental tirofiban solubility, and the relative average deviation (RAD) and the root-mean-square deviation (RMSD) were not higher than 1.18% and 4.0 × 10–6. The research on tirofiban is not only a simple measurement of the dissolution data but more importantly, with the support of these data, more thermodynamic data can be calculated to obtain tirofiban, which is an indispensable link between the improvement of the production process of tirofiban and the synthesis of pharmaceutical preparations.
中文翻译:

替罗非班在278.15–323.15 K下几种混合溶剂中的溶解度测定和建模
在测定替罗非班的溶解度期间,纯甲醇,乙醇,异丙醇和乙二醇(EG)在溶解中起主要作用;然后依次加水稀释,得到组成结构不同的分散体系,各混合液之间的浓度梯度为0.1(质量分数)。使用等温方法,将溶解度待测试的药物替罗非班加入其中,以323.15 K的高温为起始点,以5 K的间隔至278.15 K的低温间隔。压力条件不需要在普通大气压环境(101.1 kPa)下有特殊要求。结果表明,当温度接近较高水平时,在相同量的溶剂中可以溶解更多的药物。反过来,当温度接近低水平时,药物更难溶解。温度恒定,有机溶剂的比例大,分散体系的溶解能力将向正方向发展。相反,如果水的比例大,则溶解能力将降低。替罗非班晶体接受了各种溶出度数据的测定,包括那些经过X射线功率衍射(XPRD)检测的未溶化原材料的溶出度数据。获得的结果令人鼓舞,未发现该物质的晶体结构发生变化。计算了替罗非班的溶解度,使用了三种模型,分别是Jouyban-Acree模型,van't Hoff-Jouyban-Acree模型和改良的Apelblat-Jouyban-Acree模型。–6。对替罗非班的研究不仅是对溶出度数据的简单测量,而且更重要的是,在这些数据的支持下,可以计算出更多的热力学数据来获得替罗非班,这是改善替罗非班生产工艺与提高生产效率之间不可缺少的联系。药物制剂的合成。
更新日期:2020-08-14
中文翻译:

替罗非班在278.15–323.15 K下几种混合溶剂中的溶解度测定和建模
在测定替罗非班的溶解度期间,纯甲醇,乙醇,异丙醇和乙二醇(EG)在溶解中起主要作用;然后依次加水稀释,得到组成结构不同的分散体系,各混合液之间的浓度梯度为0.1(质量分数)。使用等温方法,将溶解度待测试的药物替罗非班加入其中,以323.15 K的高温为起始点,以5 K的间隔至278.15 K的低温间隔。压力条件不需要在普通大气压环境(101.1 kPa)下有特殊要求。结果表明,当温度接近较高水平时,在相同量的溶剂中可以溶解更多的药物。反过来,当温度接近低水平时,药物更难溶解。温度恒定,有机溶剂的比例大,分散体系的溶解能力将向正方向发展。相反,如果水的比例大,则溶解能力将降低。替罗非班晶体接受了各种溶出度数据的测定,包括那些经过X射线功率衍射(XPRD)检测的未溶化原材料的溶出度数据。获得的结果令人鼓舞,未发现该物质的晶体结构发生变化。计算了替罗非班的溶解度,使用了三种模型,分别是Jouyban-Acree模型,van't Hoff-Jouyban-Acree模型和改良的Apelblat-Jouyban-Acree模型。–6。对替罗非班的研究不仅是对溶出度数据的简单测量,而且更重要的是,在这些数据的支持下,可以计算出更多的热力学数据来获得替罗非班,这是改善替罗非班生产工艺与提高生产效率之间不可缺少的联系。药物制剂的合成。









































 京公网安备 11010802027423号
京公网安备 11010802027423号