当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition and Crystal Structure of the Human DHTKD1-Thiamin Diphosphate Complex.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-07-07 , DOI: 10.1021/acschembio.0c00114 João Leandro 1, 2 , Susmita Khamrui 3 , Hui Wang 3, 4 , Chalada Suebsuwong 3, 4 , Natalia S Nemeria 5 , Khoi Huynh 3, 4 , Moses Moustakim 3, 4 , Cody Secor 3 , May Wang 1, 2 , Tetyana Dodatko 1, 2 , Brandon Stauffer 6 , Christopher G Wilson 7 , Chunli Yu 1, 6 , Michelle R Arkin 7 , Frank Jordan 5 , Roberto Sanchez 3, 4 , Robert J DeVita 3, 4 , Michael B Lazarus 3 , Sander M Houten 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-07-07 , DOI: 10.1021/acschembio.0c00114 João Leandro 1, 2 , Susmita Khamrui 3 , Hui Wang 3, 4 , Chalada Suebsuwong 3, 4 , Natalia S Nemeria 5 , Khoi Huynh 3, 4 , Moses Moustakim 3, 4 , Cody Secor 3 , May Wang 1, 2 , Tetyana Dodatko 1, 2 , Brandon Stauffer 6 , Christopher G Wilson 7 , Chunli Yu 1, 6 , Michelle R Arkin 7 , Frank Jordan 5 , Roberto Sanchez 3, 4 , Robert J DeVita 3, 4 , Michael B Lazarus 3 , Sander M Houten 1, 2
Affiliation
|
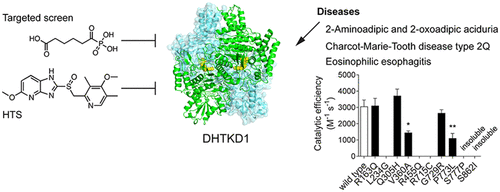
DHTKD1 is the E1 component of the 2-oxoadipate dehydrogenase complex, which is an enzyme involved in the catabolism of (hydroxy-)lysine and tryptophan. Mutations in DHTKD1 have been associated with 2-aminoadipic and 2-oxoadipic aciduria, Charcot–Marie–Tooth disease type 2Q and eosinophilic esophagitis, but the pathophysiology of these clinically distinct disorders remains elusive. Here, we report the identification of adipoylphosphonic acid and tenatoprazole as DHTKD1 inhibitors using targeted and high throughput screening, respectively. We furthermore elucidate the DHTKD1 crystal structure with thiamin diphosphate bound at 2.25 Å. We also report the impact of 10 disease-associated missense mutations on DHTKD1. Whereas the majority of the DHTKD1 variants displayed impaired folding or reduced thermal stability in combination with absent or reduced enzyme activity, three variants showed no abnormalities. Our work provides chemical and structural tools for further understanding of the function of DHTKD1 and its role in several human pathologies.
中文翻译:

人 DHTKD1-硫胺素二磷酸复合物的抑制作用和晶体结构。
DHTKD1 是 2-氧代己二酸脱氢酶复合物的 E1 组分,该复合物是一种参与(羟基-)赖氨酸和色氨酸分解代谢的酶。DHTKD1 突变与 2-氨基己二酸和 2-氧代己二酸尿症、Charcot-Marie-Tooth 病 2Q 型和嗜酸性粒细胞性食管炎有关,但这些临床上不同疾病的病理生理学仍然难以捉摸。在这里,我们报告分别使用靶向和高通量筛选鉴定己二酰膦酸和替那拉唑作为 DHTKD1 抑制剂。我们进一步阐明了硫胺二磷酸结合在 2.25 Å 处的 DHTKD1 晶体结构。我们还报告了 10 个与疾病相关的错义突变对 DHTKD1 的影响。尽管大多数 DHTKD1 变体表现出折叠受损或热稳定性降低以及酶活性缺失或降低,但三种变体未显示异常。我们的工作为进一步了解 DHTKD1 的功能及其在多种人类病理中的作用提供了化学和结构工具。
更新日期:2020-08-21
中文翻译:

人 DHTKD1-硫胺素二磷酸复合物的抑制作用和晶体结构。
DHTKD1 是 2-氧代己二酸脱氢酶复合物的 E1 组分,该复合物是一种参与(羟基-)赖氨酸和色氨酸分解代谢的酶。DHTKD1 突变与 2-氨基己二酸和 2-氧代己二酸尿症、Charcot-Marie-Tooth 病 2Q 型和嗜酸性粒细胞性食管炎有关,但这些临床上不同疾病的病理生理学仍然难以捉摸。在这里,我们报告分别使用靶向和高通量筛选鉴定己二酰膦酸和替那拉唑作为 DHTKD1 抑制剂。我们进一步阐明了硫胺二磷酸结合在 2.25 Å 处的 DHTKD1 晶体结构。我们还报告了 10 个与疾病相关的错义突变对 DHTKD1 的影响。尽管大多数 DHTKD1 变体表现出折叠受损或热稳定性降低以及酶活性缺失或降低,但三种变体未显示异常。我们的工作为进一步了解 DHTKD1 的功能及其在多种人类病理中的作用提供了化学和结构工具。







































 京公网安备 11010802027423号
京公网安备 11010802027423号