当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Graphdiyne and Hydrogen-Substituted Graphdiyne as Potential Cathode Materials for High-Capacity Aluminum-Ion Batteries
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-07-06 00:00:00 , DOI: 10.1021/acsaem.0c00805 Shaikat Debnath 1 , Calvin Phan 1, 2 , Debra J. Searles 1, 3 , Marlies Hankel 1
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-07-06 00:00:00 , DOI: 10.1021/acsaem.0c00805 Shaikat Debnath 1 , Calvin Phan 1, 2 , Debra J. Searles 1, 3 , Marlies Hankel 1
Affiliation
|
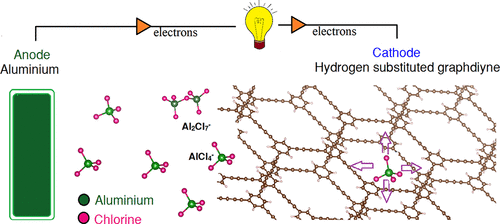
In chloroaluminate ion based aluminum-ion batteries, a graphite cathode shows promising electrochemical performance. However, its low capacity and high volume expansion is a major hindrance. This is because of the limited intercalation of the AlCl4– ion into the graphite layers. As a possible solution to this, graphdiyne (GDY) and hydrogen-substituted graphdiyne (HsGDY), two-dimensional carbon allotropes with large pores, are tested here as potential cathode materials by density functional theory calculations. We find that both materials exhibit better performance than graphite, in terms of easiness of AlCl4 intercalation, voltage profile, and storage capacity. To ensure AlCl4 intercalation during charging, the cathode materials must expand to accommodate the AlCl4. The energy required for the expansion is defined as the distortion energy. Both GDY and HsGDY require a lower distortion energy (0.006 and 0.003 eV/Å2, respectively) than graphite (0.014 eV/Å2) meaning it will be easier for the AlCl4 to intercalate into the cathode during charging. The average voltage for the AlCl4 intercalated single layer of GDY and HsGDY is around 2.15 V, which is higher than that of graphite (2 V). Finally, the storage capacity of the bilayer system of GDY and HsGDY based on stage-1 storage (AlCl4 intercalation between every layer of the cathode material) is 124 and 456 mA h g–1, respectively; whereas a bilayer graphene system has a capacity of 155 mA h g–1. Therefore, HsGDY has a significantly higher storage capacity than graphite.
中文翻译:

Graphdiyne和氢取代的Graphdiyne作为高容量铝离子电池的潜在阴极材料
在基于氯铝酸盐离子的铝离子电池中,石墨阴极显示出有希望的电化学性能。但是,其低容量和高容量扩展是主要障碍。这是因为的AlCl有限嵌入的4 -离子进入石墨层。作为对此的可能解决方案,此处通过密度泛函理论计算将具有大孔的二维碳同素异形体石墨二炔(GDY)和氢取代的石墨二炔(HsGDY)作为潜在的阴极材料进行了测试。我们发现,就AlCl 4嵌入的难易程度,电压分布和存储容量而言,这两种材料均表现出比石墨更好的性能。确保AlCl 4在充电期间插入,阴极材料必须膨胀以容纳AlCl 4。膨胀所需的能量定义为畸变能量。既GDY和HsGDY需要较低失真的能量(0.006和0.003伏特/ A 2,分别地)比石墨(0.014电子伏/ A 2),这意味着它会更容易为的AlCl 4在充电过程中嵌插到阴极。GDY和HsGDY的AlCl 4插层单层的平均电压约为2.15 V,高于石墨(2 V)的平均电压。最后,基于第一阶段存储(AlCl 4)的GDY和HsGDY双层系统的存储容量阴极材料每一层之间的插层分别为124和456 mA hg –1;而双层石墨烯系统的容量为155 mA hg –1。因此,HsGDY具有比石墨高得多的存储容量。
更新日期:2020-07-06
中文翻译:

Graphdiyne和氢取代的Graphdiyne作为高容量铝离子电池的潜在阴极材料
在基于氯铝酸盐离子的铝离子电池中,石墨阴极显示出有希望的电化学性能。但是,其低容量和高容量扩展是主要障碍。这是因为的AlCl有限嵌入的4 -离子进入石墨层。作为对此的可能解决方案,此处通过密度泛函理论计算将具有大孔的二维碳同素异形体石墨二炔(GDY)和氢取代的石墨二炔(HsGDY)作为潜在的阴极材料进行了测试。我们发现,就AlCl 4嵌入的难易程度,电压分布和存储容量而言,这两种材料均表现出比石墨更好的性能。确保AlCl 4在充电期间插入,阴极材料必须膨胀以容纳AlCl 4。膨胀所需的能量定义为畸变能量。既GDY和HsGDY需要较低失真的能量(0.006和0.003伏特/ A 2,分别地)比石墨(0.014电子伏/ A 2),这意味着它会更容易为的AlCl 4在充电过程中嵌插到阴极。GDY和HsGDY的AlCl 4插层单层的平均电压约为2.15 V,高于石墨(2 V)的平均电压。最后,基于第一阶段存储(AlCl 4)的GDY和HsGDY双层系统的存储容量阴极材料每一层之间的插层分别为124和456 mA hg –1;而双层石墨烯系统的容量为155 mA hg –1。因此,HsGDY具有比石墨高得多的存储容量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号