Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microglia modulate gliotransmission through the regulation of VAMP2 proteins in astrocytes.
Glia ( IF 5.4 ) Pub Date : 2020-07-07 , DOI: 10.1002/glia.23884 Fuyuko Takata-Tsuji 1, 2, 3 , Naura Chounlamountri 1, 2 , Le-Duy Do 1, 2 , Camille Philippot 1, 2 , Julia Novion Ducassou 4 , Yohann Couté 4 , Sarrah Ben Achour 5 , Jérôme Honnorat 1, 2, 6 , Christophe Place 7 , Olivier Pascual 1, 2
Glia ( IF 5.4 ) Pub Date : 2020-07-07 , DOI: 10.1002/glia.23884 Fuyuko Takata-Tsuji 1, 2, 3 , Naura Chounlamountri 1, 2 , Le-Duy Do 1, 2 , Camille Philippot 1, 2 , Julia Novion Ducassou 4 , Yohann Couté 4 , Sarrah Ben Achour 5 , Jérôme Honnorat 1, 2, 6 , Christophe Place 7 , Olivier Pascual 1, 2
Affiliation
|
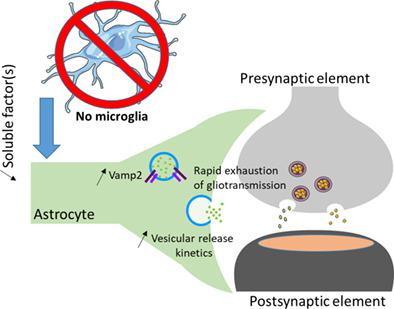
Vesicular release is one of the release mechanisms of various signaling molecules. In neurons, the molecular machinery involved in vesicular release has been designed through evolution to trigger fast and synchronous release of neurotransmitters. Similar machinery with a slower kinetic and a slightly different molecular assembly allows astrocytes to release various transmitters such as adenosine triphosphate (ATP), glutamate, and D‐serine. Astrocytes are important modulators of neurotransmission through gliotransmitter release. We recently demonstrated that microglia, another type of glia, release ATP to modulate synaptic transmission using astrocytes as intermediate. We now report that microglia regulate astrocytic gliotransmission through the regulation of SNARE proteins in astrocytes. Indeed, we found that gliotransmission triggered by P2Y1 agonist is impaired in slices from transgenic mice devoid of microglia. Using total internal reflection fluorescence imaging, we found that the vesicular release of gliotransmitter by astrocytes was different in cultures lacking microglia compared to vesicular release in astrocytes cocultured with microglia. Quantification of the kinetic of vesicular release indicates that the overall release appears to be faster in pure astrocyte cultures with more vesicles close to the membrane when compared to astrocytes cocultured with microglia. Finally, biochemical investigation of SNARE protein expression indicates an upregulation of VAMP2 in absence of microglia. Altogether, these results indicate that microglia seems to be involved in the regulation of an astrocytic phenotype compatible with proper gliotransmission. The mechanisms described in this study could be of importance for central nervous system diseases where microglia are activated.
中文翻译:

小胶质细胞通过调节星形胶质细胞中的 VAMP2 蛋白来调节胶质传递。
囊泡释放是各种信号分子的释放机制之一。在神经元中,参与囊泡释放的分子机制已通过进化设计,以触发神经递质的快速和同步释放。具有较慢动力学和稍微不同的分子组装的类似机制允许星形胶质细胞释放各种递质,例如三磷酸腺苷 (ATP)、谷氨酸和 D-丝氨酸。星形胶质细胞是通过释放胶质递质进行神经传递的重要调节剂。我们最近证明,小胶质细胞是另一种类型的胶质细胞,它使用星形胶质细胞作为中间体释放 ATP 以调节突触传递。我们现在报告小胶质细胞通过调节星形胶质细胞中的 SNARE 蛋白来调节星形胶质细胞的胶质传递。的确,我们发现由 P2Y1 激动剂触发的胶质传递在没有小胶质细胞的转基因小鼠的切片中受损。使用全内反射荧光成像,我们发现星形胶质细胞释放的胶质递质在缺乏小胶质细胞的培养物中与与小胶质细胞共培养的星形胶质细胞的囊泡释放相比是不同的。囊泡释放动力学的量化表明,与与小胶质细胞共培养的星形胶质细胞相比,在具有更多靠近膜的囊泡的纯星形胶质细胞培养物中,整体释放似乎更快。最后,SNARE 蛋白表达的生化研究表明 VAMP2 在没有小胶质细胞的情况下上调。共,这些结果表明,小胶质细胞似乎参与了与适当的胶质传递相容的星形胶质细胞表型的调节。本研究中描述的机制对于小胶质细胞被激活的中枢神经系统疾病可能很重要。
更新日期:2020-07-07
中文翻译:

小胶质细胞通过调节星形胶质细胞中的 VAMP2 蛋白来调节胶质传递。
囊泡释放是各种信号分子的释放机制之一。在神经元中,参与囊泡释放的分子机制已通过进化设计,以触发神经递质的快速和同步释放。具有较慢动力学和稍微不同的分子组装的类似机制允许星形胶质细胞释放各种递质,例如三磷酸腺苷 (ATP)、谷氨酸和 D-丝氨酸。星形胶质细胞是通过释放胶质递质进行神经传递的重要调节剂。我们最近证明,小胶质细胞是另一种类型的胶质细胞,它使用星形胶质细胞作为中间体释放 ATP 以调节突触传递。我们现在报告小胶质细胞通过调节星形胶质细胞中的 SNARE 蛋白来调节星形胶质细胞的胶质传递。的确,我们发现由 P2Y1 激动剂触发的胶质传递在没有小胶质细胞的转基因小鼠的切片中受损。使用全内反射荧光成像,我们发现星形胶质细胞释放的胶质递质在缺乏小胶质细胞的培养物中与与小胶质细胞共培养的星形胶质细胞的囊泡释放相比是不同的。囊泡释放动力学的量化表明,与与小胶质细胞共培养的星形胶质细胞相比,在具有更多靠近膜的囊泡的纯星形胶质细胞培养物中,整体释放似乎更快。最后,SNARE 蛋白表达的生化研究表明 VAMP2 在没有小胶质细胞的情况下上调。共,这些结果表明,小胶质细胞似乎参与了与适当的胶质传递相容的星形胶质细胞表型的调节。本研究中描述的机制对于小胶质细胞被激活的中枢神经系统疾病可能很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号