当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Overcoming vincristine resistance in cancer: Computational design and discovery of piperine-inspired P-glycoprotein inhibitors.
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-07-07 , DOI: 10.1111/cbdd.13758 Safiulla Basha Syed,Shu-Yu Lin,Hemant Arya,I-Hsuan Fu,Teng-Kuang Yeh,Mariasoosai Ramya Chandar Charles,Latha Periyasamy,Hsing-Pang Hsieh,Mohane Selvaraj Coumar
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-07-07 , DOI: 10.1111/cbdd.13758 Safiulla Basha Syed,Shu-Yu Lin,Hemant Arya,I-Hsuan Fu,Teng-Kuang Yeh,Mariasoosai Ramya Chandar Charles,Latha Periyasamy,Hsing-Pang Hsieh,Mohane Selvaraj Coumar
|
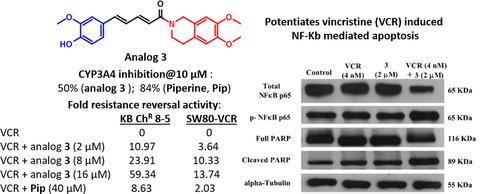
P‐glycoprotein (P‐gp)/MDR‐1 plays a major role in the development of multidrug resistance (MDR) by pumping the chemotherapeutic drugs out of the cancer cells and reducing their efficacy. A number of P‐gp inhibitors were reported to reverse the MDR when co‐administered with chemotherapeutic drugs. Unfortunately, none has approved for clinical use due to toxicity issues. Some of the P‐gp inhibitors tested in the clinics are reported to have cross‐reactivity with CYP450 drug‐metabolizing enzymes, resulting in unpredictable pharmacokinetics and toxicity of co‐administered chemotherapeutic drugs. In this study, two piperine analogs (3 and 4) having lower cross‐reactivity with CYP3A4 drug‐metabolizing enzyme are identified as P‐glycoprotein (P‐gp) inhibitors through computational design, followed by synthesis and testing in MDR cancer cell lines over‐expressing P‐gp (KB ChR 8–5, SW480‐VCR, and HCT‐15). Both the analogs significantly increased the vincristine efficacy in MDR cancer cell lines at low micromole concentrations. Specifically, 3 caused complete reversal of vincristine resistance in KB ChR 8–5 cells and found to act as competitive inhibitor of P‐gp as well as potentiated the vincristine‐induced NF‐KB‐mediated apoptosis. Therefore, 3 ((2E,4E)‐1‐(6,7‐dimethoxy‐3,4‐dihydroisoquinolin‐2(1H)‐yl)‐5‐(4‐hydroxy‐3‐methoxyphenyl)penta‐2,4‐dien‐1‐one) can serve as a potential P‐gp inhibitor for in vivo investigations, to reverse multidrug resistance in cancer.
中文翻译:

克服癌症中的长春新碱耐药性:胡椒碱启发的 P-糖蛋白抑制剂的计算设计和发现。
P-糖蛋白 (P-gp)/MDR-1 通过将化疗药物泵出癌细胞并降低其疗效,在多药耐药性 (MDR) 的发展中起主要作用。据报道,当与化疗药物共同给药时,许多 P-gp 抑制剂可以逆转 MDR。不幸的是,由于毒性问题,没有一个被批准用于临床。据报道,在临床中测试的一些 P-gp 抑制剂与 CYP450 药物代谢酶有交叉反应,导致联合给药的化疗药物的药代动力学和毒性不可预测。在本研究中,两种胡椒碱类似物(3和4) 与 CYP3A4 药物代谢酶具有较低的交叉反应性,通过计算设计被鉴定为 P-糖蛋白 (P-gp) 抑制剂,然后在过表达 P-gp 的 MDR 癌细胞系中进行合成和测试 (KB Ch R 8– 5、SW480-VCR 和 HCT-15)。在低微摩尔浓度下,这两种类似物都显着提高了长春新碱在 MDR 癌细胞系中的功效。具体而言,3导致 KB Ch R 8-5 细胞中长春新碱耐药性的完全逆转,并发现它可作为 P-gp 的竞争性抑制剂并增强长春新碱诱导的 NF-KB 介导的细胞凋亡。因此,3 ((2 E ,4 E )-1-(6,7-二甲氧基-3,4-二氢异喹啉-2(1 H)-yl)-5-(4-羟基-3-甲氧基苯基)penta-2,4-dien-1-one) 可作为体内研究的潜在 P-gp 抑制剂,以逆转癌症的多药耐药性。
更新日期:2020-07-07
中文翻译:

克服癌症中的长春新碱耐药性:胡椒碱启发的 P-糖蛋白抑制剂的计算设计和发现。
P-糖蛋白 (P-gp)/MDR-1 通过将化疗药物泵出癌细胞并降低其疗效,在多药耐药性 (MDR) 的发展中起主要作用。据报道,当与化疗药物共同给药时,许多 P-gp 抑制剂可以逆转 MDR。不幸的是,由于毒性问题,没有一个被批准用于临床。据报道,在临床中测试的一些 P-gp 抑制剂与 CYP450 药物代谢酶有交叉反应,导致联合给药的化疗药物的药代动力学和毒性不可预测。在本研究中,两种胡椒碱类似物(3和4) 与 CYP3A4 药物代谢酶具有较低的交叉反应性,通过计算设计被鉴定为 P-糖蛋白 (P-gp) 抑制剂,然后在过表达 P-gp 的 MDR 癌细胞系中进行合成和测试 (KB Ch R 8– 5、SW480-VCR 和 HCT-15)。在低微摩尔浓度下,这两种类似物都显着提高了长春新碱在 MDR 癌细胞系中的功效。具体而言,3导致 KB Ch R 8-5 细胞中长春新碱耐药性的完全逆转,并发现它可作为 P-gp 的竞争性抑制剂并增强长春新碱诱导的 NF-KB 介导的细胞凋亡。因此,3 ((2 E ,4 E )-1-(6,7-二甲氧基-3,4-二氢异喹啉-2(1 H)-yl)-5-(4-羟基-3-甲氧基苯基)penta-2,4-dien-1-one) 可作为体内研究的潜在 P-gp 抑制剂,以逆转癌症的多药耐药性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号