Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.mcat.2020.111088 Shuxue Yang , Gang Yang
|
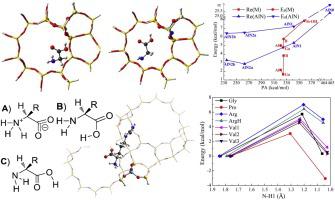
Adsorption and isomerization of amino acids within zeolites are investigated by two-layer ONIOM theoretical methods, focusing on the impacts of acidity, amine functionalization, pore topology and sidechains. Meanwhile, relationships of zwitterionic stabilization and isomerization activities with zeolite acidity and basicity are established. Adsorption structures are strongly perturbed by all factors except acidity. Distribution of different glycine isomers is significantly altered by all zeolites and also depends on zeolite basicity. Zwitterionic isomers, the active form to conduct the biological and pharmaceutical functions, are non-existent in the isolated state while remain stable within all acidic zeolites or not very highly basic zeolites. Zwitterions always show stronger interactions with zeolites than canonical isomers and zeolites with moderate acidities or basicities are the most effective for zwitterionic stabilization. Larger porous zeolites favor zwitterionic stabilization, and distinct from the isolated state, sidechains disfavor zwitterionic stabilization within zeolites while degree of influences depends on identity of sidechains, causing relative stabilities with canonical isomers to rank as proline (Pro) > glycine (Gly) > valine (Val) > protonated arginine (ArgH) > arginine (Arg). Zeolites greatly stabilize zwitterions and enable the occurrence of catalytic isomerization reactions. Isomerization to zwitterionic isomers proceeds facilely within all zeolites. Acidity increase of M-ZSM-5 zeolites or basicity decrease of amine-functionalized zeolites facilitates zwitterion formation, and zwitterion formation is the most favorable for Pro while disfavored for Arg. Results also provide insights to other host-guest interactions and catalytic mechanisms.
中文翻译:

沸石中氨基酸的吸附和异构化:酸度,胺官能化,孔拓扑和侧链的影响
通过两层ONIOM理论方法研究了沸石中氨基酸的吸附和异构化,重点是酸度,胺功能化,孔拓扑和侧链的影响。同时,建立了两性离子稳定和异构化活性与沸石酸度和碱度的关系。吸附结构会受到除酸度以外的所有因素的强烈干扰。所有沸石都会显着改变不同甘氨酸异构体的分布,并且还取决于沸石的碱性。两性离子异构体是一种具有生物学和药物功能的活性形式,在分离状态下不存在,而在所有酸性沸石或不是很强碱性的沸石中都保持稳定。两性离子总是显示出比规范异构体更强的与沸石的相互作用,具有中等酸度或碱度的沸石对于两性离子稳定化最有效。较大的多孔沸石有利于两性离子稳定,并且不同于分离状态,侧链不利于沸石内的两性离子稳定,而影响程度取决于侧链的身份,从而导致典型异构体的相对稳定性为脯氨酸(Pro)>甘氨酸(Gly)>缬氨酸。 (Val)>质子化精氨酸(ArgH)>精氨酸(Arg)。沸石极大地稳定了两性离子并使得能够发生催化异构化反应。在所有沸石中,容易进行异构化为两性离子异构体。M-ZSM-5沸石的酸度增加或胺官能化沸石的碱度降低促进两性离子形成,而两性离子形成对Pro最有利,而对Arg不有利。结果还提供了其他宿主-客体相互作用和催化机制的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号