Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.mcat.2020.111086 E. Gonzalez-A , R. Rangel , A. Solís-Garcia , A.M. Venezia , T.A. Zepeda
|
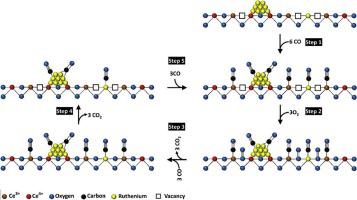
New insight on the understanding of the reaction mechanisms during the CO oxidation over Ru-containing CeO2 catalysts was reported. A set of ruthenium high surface area CeO2 oxides, with two different Ru loadings, was prepared for studying the evolution of Ru and Ce species during CO oxidation reaction, using a reaction mixture of 1 % CO, 0.5 % O2, N2 balance and a contact time W/FCO = 7.4 gcat h molCO−1. The samples were characterized by SBET, XRD, DRS UV–vis, TPR, Raman spectroscopy, and XPS. Furthermore, the samples were investigated by FTIR measurements during CO oxidation under reaction conditions. The catalysts with low Ru loading (1.5 wt.%) presented higher catalytic activity than those containing a higher Ru loading (3 % wt.). The catalytic activity test results strongly depend on electronic changes observed at the interface/surface of ceria support. The characterization results and FTIR investigation during CO oxidation show that the presence of ruthenium species modifies the electronic properties of the ceria surface promoting a change at their surface where a redox-mechanism was detected. This causes the formation of surface oxygen vacancies which promotes oxygen mobility from the ceria lattice contributing to the CO oxidation. The ruthenium incorporation drastically favors the CO adsorption (carbonyl and dicarbonyl species). However, our FTIR data not shown a direct correlation between the ruthenium-carbonyl species (linear and bridged form) on the CO transformation. Further, the presence of ruthenium enhances the formation of surface oxygen vacancies, which favor the oxygen mobility from the ceria lattice toward the CO oxidation. It was found that at low temperatures (close down to the maximum conversion), the CO oxidation preferably happened on oxygen vacancy sites.
中文翻译:

Ru (x) -CeO 2催化剂在CO氧化反应条件下的FTIR研究
报道了在含Ru的CeO 2催化剂上CO氧化过程中对反应机理的理解的新见解。制备了一组具有两种不同Ru含量的钌高表面积CeO 2氧化物,用于研究1%CO,0.5%O 2,N 2平衡的反应混合物中Ru和Ce物种在CO氧化反应过程中的演变。和接触时间W / F CO = 7.4 g cat h mol CO -1。样品通过S BET表征,XRD,DRS紫外可见,TPR,拉曼光谱和XPS。此外,通过在反应条件下CO氧化期间的FTIR测量来研究样品。具有低Ru负载量(1.5重量%)的催化剂表现出比具有较高Ru负载量(3重量%)的催化剂更高的催化活性。催化活性测试结果在很大程度上取决于在二氧化铈载体界面/表面观察到的电子变化。表征结果和在CO氧化过程中的FTIR研究表明,钌物种的存在改变了二氧化铈表面的电子性能,从而促进了氧化还原机理在其表面的变化。这导致形成表面氧空位,其促进二氧化铈晶格中的氧迁移,从而有助于CO氧化。钌的掺入极大地促进了CO的吸附(羰基和二羰基物质)。但是,我们的FTIR数据并未显示出CO转化过程中的羰基钌(线性和桥接形式)之间存在直接关系。此外,钌的存在增强了表面氧空位的形成,这有利于从二氧化铈晶格向CO氧化的氧迁移率。发现在低温下(接近最大转化率),CO氧化优选发生在氧空位上。有利于从二氧化铈晶格向CO氧化的氧迁移率。发现在低温下(接近最大转化率),CO氧化优选发生在氧空位上。有利于从二氧化铈晶格向CO氧化的氧迁移率。发现在低温下(接近最大转化率),CO氧化优选发生在氧空位上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号