Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.mcat.2020.111090 Shiyang Wang , Boping Liu , Yulong Jin
|
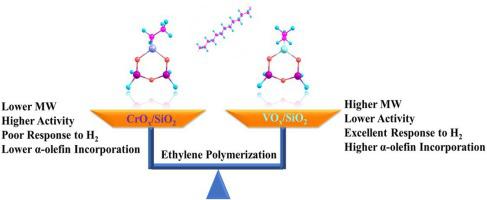
Phillips catalyst (CrOx/SiO2) is one of the most famous industrial catalysts for ethylene polymerization, while VOx/SiO2 catalyst is a promising catalyst capable of making UHMWPE, which was recently developed in our group. These two catalysts were known to show completely deferent behaviors in ethylene polymerization in terms of activity, H2 response and copolymerization ability. However, a molecular insight into the mechanism is not well understood. Herein, a theoretical comparison between them via the propagation through insertion and termination through chain transfer reaction pathways is performed. The results show the transition states for olefins (ethylene, 1-butene and 1-hexene) insertion are energetically more stable in VOx/SiO2. However, the binding energies of the olefins by VOx/SiO2 is obviously lower, which results in the insertion barriers for VOx/SiO2 about 2–3 kcal mol−1 higher than those for CrOx/SiO2. As to the termination, CrOx/SiO2 is more probably to terminate via the BHE (β-H elimination to Cr center) pathway, which generally has lower barriers than those for BHT (β-H elimination to monomer) in the VOx/SiO2 system. Meanwhile, VOx/SiO2 is superior to CrOx/SiO2 in hydrogenolysis, because it is more accessible by the H2, and the reaction barrier is also much lower. The energy gap between the barriers for propagation and termination is generally smaller for CrOx/SiO2, implying its tendency to produce lower MW PE when compared with VOx/SiO2. Furthermore, for CrOx/SiO2, the insertion barriers between C2H4 and α-olefin are bigger and the promotion effect of the incorporated α-olefins on termination is more obvious, which might account for the lower incorporation efficiency for comonomer by CrOx/SiO2, despite of its lower insertion barriers.
中文翻译:

为什么CrO x / SiO 2和VO x / SiO 2催化剂在乙烯聚合反应中表现出如此不同的行为?理论方法
Phillips催化剂(CrO x / SiO 2)是最著名的乙烯聚合工业催化剂之一,而VO x / SiO 2催化剂是能够制造UHMWPE的有前途的催化剂,这是我们小组最近开发的。已知这两种催化剂在乙烯聚合中表现出完全不同的活性H 2行为。反应和共聚能力。然而,对该机制的分子认识尚不十分清楚。在本文中,通过经由插入的传播和通过链转移反应途径的终止进行了它们之间的理论比较。结果表明,烯烃(乙烯,1-丁烯和1-己烯)插入的过渡态在VO x / SiO 2中在能量上更稳定。但是,VO x / SiO 2与烯烃的结合能明显较低,这导致VO x / SiO 2的插入势垒比CrO x / SiO 2的插入势垒高2-3 kcal mol -1。。至于终止作用,CrO x / SiO 2更有可能通过BHE(β-H消除至Cr中心)途径终止,该途径通常比VO x中BHT(β-H消除至单体)的阻挡层低。/ SiO 2系统。同时,在氢解中,VO x / SiO 2优于CrO x / SiO 2,因为它更容易被H 2接近,并且反应势垒也低得多。对于CrO x / SiO 2,传播和终止壁垒之间的能隙通常较小,这意味着与VO x相比,它倾向于产生更低的MW PE。/ SiO 2。此外,对于CrO x / SiO 2而言,C 2 H 4与α-烯烃之间的插入势垒更大,并且引入的α-烯烃对端基的促进作用更加明显,这可能解释了共聚单体的引入效率较低。 CrO x / SiO 2,尽管其插入势垒较低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号