Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.jmgm.2020.107678 Dharma Rao Tompa 1 , Sureshan Muthusamy 1 , Srimari Srikanth 1 , Saraboji Kadhirvel 1
|
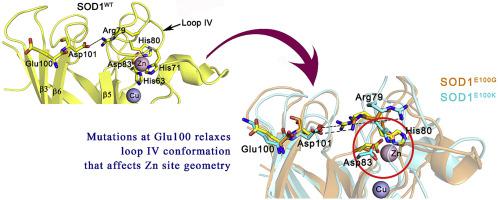
Cu/Zn superoxide dismutase (SOD1) mutations are associated to the motor neuron disorder, amyotrophic lateral sclerosis (ALS), which is characterized by aggregates of the misfolded proteins. The distribution of mutations all over the three-dimensional structure of SOD1 makes it complex to determine the exact molecular mechanism underlying SOD1 destabilization and the associated ALS pathology. In this study, we have examined structure and dynamics of SOD1 protein upon two ALS associated point mutations at the surface residue Glu100 (E100G and E100K), which is located far from the Cu and Zn sites and dimer interface. The molecular dynamics simulations were performed for these mutants for 50ns using GROMACS package. Our results indicate that the mutations result in structural destabilization by affecting the gate keeping role of Glu100 and loss of electrostatic interactions on the protein surface which stabilizes the β-barrel structure of the native form. Further, these mutations could increase the fluctuations in the zinc-binding loop (loop IV), primarily due to loss of hydrogen bond between Asp101 and Arg79. The relaxed conformation of Arg79 further affects the native conformation of His80 and Asp83, that results in altered zinc site geometry and the structure of the substrate channel. Our results clearly suggest that, similar to the mutations located at metal sites/dimer interface/disulfide regions, the mutations at the far positioned site (Glu100) also induce significant conformational changes that could affect the metallation and structure of SOD1 molecule, resulting in formation of toxic intermediate species that cause ALS.
中文翻译:

Cu/Zn SOD1 远定位表面突变的分子动力学促进了结构稳定性和金属结合位点的改变:肌萎缩侧索硬化发病机制的结构线索。
铜/锌超氧化物歧化酶 (SOD1) 突变与运动神经元疾病、肌萎缩侧索硬化症 (ALS) 相关,其特征是错误折叠的蛋白质聚集。SOD1 整个三维结构中的突变分布使得确定 SOD1 不稳定和相关 ALS 病理学的确切分子机制变得复杂。在这项研究中,我们检查了 SOD1 蛋白在表面残基 Glu100(E100G 和 E100K)的两个与 ALS 相关的点突变后的结构和动力学,其位于远离铜和锌位点和二聚体界面的位置。使用 GROMACS 包对这些突变体进行 50ns 的分子动力学模拟。我们的结果表明,突变通过影响 Glu100 的门控作用和蛋白质表面静电相互作用的丧失导致结构不稳定,从而稳定了天然形式的 β-桶结构。此外,这些突变可能会增加锌结合环(环 IV)的波动,主要是由于 Asp101 和 Arg79 之间的氢键丧失。Arg79 的松弛构象进一步影响 His80 和 Asp83 的天然构象,导致锌位点几何结构和底物通道结构发生改变。我们的结果清楚地表明,类似于位于金属位点/二聚体界面/二硫键区域的突变,











































 京公网安备 11010802027423号
京公网安备 11010802027423号