Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.jcat.2020.06.040 Kenji Taira , Takeharu Sugiyama , Hisahiro Einaga , Kenji Nakao , Kimihito Suzuki
|
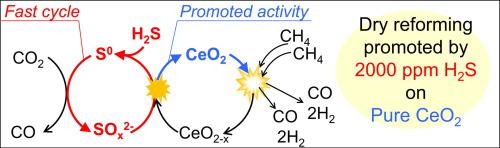
Biomass-derived gas and unused industrial byproducts are resources for H2 production with low CO2 emission; however, they contain > 1000 ppm H2S, which necessitates the use of H2S-tolerant catalysts. Herein, a pure CeO2 catalyst demonstrated stable and efficient H2 production via the dry reforming reaction in the presence of 2000 ppm H2S. No impurity phases and carbon deposition appeared on the catalyst after the reaction. Moreover, CeO2 catalytic activity improved with H2S concentration. The effect of H2S on CeO2 was determined by in situ infrared spectroscopy and S K-edge X-ray absorption near-edge structure studies. The re-oxidation of CeO2 by CO2, a key step of the dry reforming reaction, was accelerated by the rapid redox cycle of sulfur, which promoted CH4 activation and decreased the apparent activation energy from 125 to 101 kJ/mol. The redox cycle of S made the pure CeO2 catalyst resistant to high concentration of H2S.
中文翻译:

2000 ppm H 2 S对CH 4在纯CeO 2上的干重整反应的促进作用以及反应过程中硫行为的原位观察
生物质衍生的气体和未使用的工业副产品是用于生产H 2的资源,其CO 2排放低。但是,它们含有> 1000 ppm的H 2 S,因此必须使用耐H 2 S的催化剂。在此,纯的CeO 2催化剂在2000ppm H 2 S的存在下通过干重整反应表现出稳定且有效的H 2产生。反应后,催化剂上没有出现杂质相和碳沉积。此外,CeO 2的催化活性随H 2 S浓度的提高而提高。H 2 S对CeO 2的影响通过原位红外光谱和S K边缘X射线吸收近边缘结构研究确定。硫的快速氧化还原循环促进了干重整反应关键步骤CeO 2被CO 2的再氧化,从而促进了CH 4的活化并将表观活化能从125 kJ / mol降低。S的氧化还原循环使纯CeO 2催化剂对高浓度的H 2 S具有抗性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号