Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.jcat.2020.06.035 Neil K. Razdan , Aditya Bhan
|
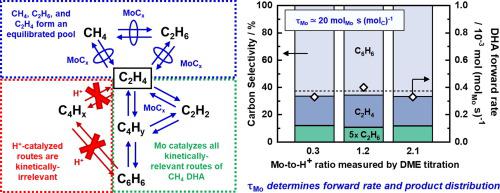
Systematic variation of Mo/Al = {0.10, 0.25, 0.35} in Mo/H-ZSM-5 catalysts effects ~20× change of Mo-to-H+ ratio during methane dehydroaromatization (DHA) reactions at steady state and on deactivating catalysts. Mo and Brønsted-acid site count, respectively enumerated by ethane hydrogenolysis probe reaction and dimethyl ether chemical titration, evolve disparately during DHA catalysis, indicating the two sites reduce in number by distinct mechanisms. The deactivation of Mo/H-ZSM-5 catalytic activity is uniquely attributed to the loss of active, or accessible, Mo sites, evinced by (i) the non-selective nature of deactivation (i.e. that deactivation occurs without modification of residual active sites), (ii) invariance in product distribution from Mo/H+ = 0.10–2.1, and (iii) linear correspondence between Mo-catalyzed ethane hydrogenolysis rate and active site count during DHA on deactivating samples. DHA forward rate – the intrinsic kinetic descriptor of catalyst activity – normalizes by Mo content at steady state and on deactivating catalysts with Mo/Al = 0.10–0.35 for Mo-based contact times in the range τMo = 0.79–44 molMo s mol−1C, unequivocally establishing Mo aggregates as the sole kinetically-relevant active site on Mo/H-ZSM-5. Brønsted-acid site content over a ~40× range in τH+ = 1.4–54 molH+ s mol−1C has no discernable correlation with or effect on DHA rate, methane conversion, or product distribution – demonstrating H+ does not catalyze any rate- or selectivity-determining steps in the benzene formation pathway and suggesting that the benefits of zeolitic acid sites are only to disperse Mo during catalyst synthesis and, conceivably, to catalyze equilibrated reaction steps.
中文翻译:

Carbidic Mo是Mo / H-ZSM-5上甲烷甲烷脱氢芳构化的唯一动力学相关活性位点
Mo / H-ZSM-5催化剂中Mo / Al = {0.10,0.25,0.35}的系统变化影响甲烷脱氢芳构化(DHA)反应在稳态和失活催化剂上Mo-H +比的〜20倍变化。乙烷氢解探针反应和二甲醚化学滴定分别列举的Mo和Brønsted-酸位点数,在DHA催化过程中会完全不同地发展,表明这两个位点的数量因不同的机理而减少。Mo / H-ZSM-5催化活性的失活独特地归因于活性或可及的Mo位置的丧失,具体表现为(i)失活的非选择性性质(即失活发生时未修饰残留的活性位点),(ii)Mo / H += 0.10–2.1,以及(iii)失活样品上DHA期间Mo催化的乙烷氢解速率与活性位点之间的线性对应关系。DHA正向速率-催化剂活性的本征动力学描述符-通过在稳定状态下的Mo含量和对τ的范围内停用与钼/铝= 0.10-0.35催化剂基于Mo的接触时间规格化沫 = 0.79-44摩尔沫小号摩尔-1 C,明确地确定Mo聚集体为Mo / H-ZSM-5上唯一的动力学相关活性位点。布朗斯台德酸位点含量在τH + = 1.4–54 mol H + s mol -1 C的〜40x范围内与DHA速率,甲烷转化率或产物分布没有明显的相关关系或对DHA速率,甲烷转化率或产物分布没有影响-表明H +不会催化苯形成途径中任何决定速率或选择性的步骤,并表明沸石酸位的益处仅是分散Mo在催化剂合成过程中,并且可以想到地催化平衡的反应步骤。










































 京公网安备 11010802027423号
京公网安备 11010802027423号