European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.ejmech.2020.112499 Bin Xiao 1 , Chun Zhang 1 , Xiaotong Song 1 , Miao Wu 1 , Jianping Mao 1 , Rong Yu 1 , Yongxiang Zheng 1
|
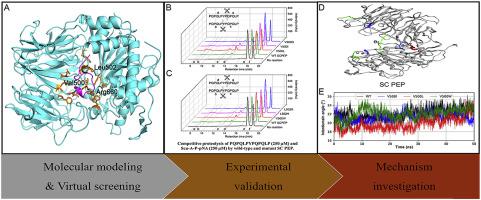
Celiac disease affects approximately 1% of the population and is a major public health problem worldwide. It is trigged by gluten-derived peptides, which have unusually high proline-glutamine motif content and are highly resistant to proteolysis by digestive enzymes of the gastrointestinal tract. The only treatment for celiac disease is strict, lifelong adherence to a gluten-free diet, which is effective but costly and difficult to maintain. Therefore, novel non-dietary therapies for celiac disease are urgently needed. Gluten-degrading enzymes are promising non-dietary treatments, and some enzymes have been investigated in preclinical or clinical studies. A combination of prolyl endopeptidase from Sphingomonas capsulata (SC PEP) and a glutamine-specific endoprotease (EP-B2 from barley) known as latiglutenase showed insufficient benefits in phase II clinical trials, likely because of its low enzyme activity in the gastric environment. Therefore, improving enzyme activity is essential for the clinical application of SC PEP. Enzyme activity can be enhanced using computer-aided rational protein design tools. In this study, we combined molecular docking and molecular dynamics simulation to rationally design SC PEP mutants and experimentally evaluated their activities. We identified mutants with up to 90–103% increases in specific activity and up to 80–202% increases in the catalytic rate. We have investigated the mechanism underlying the enhanced activity of these mutants, and found that a conformational transition of the β-propeller domain and catalytic domain of SC PEP was important for enzyme activity, and this transition was affected by residues in the catalytic domain and at the domain interface; a shorter distance between the substrate Pro and the oxyanion holes was also crucial for improving SC PEP catalytic activity. Our results provide useful information for the rational design of highly active SC PEPs to accelerate the development of enzyme therapeutics candidates for Celiac disease.
中文翻译:

来自荚膜鞘氨醇单胞菌的工程改造的脯氨酰内肽酶具有改善的对乳糜泻的病原性肽的水解活性。
腹腔疾病影响大约1%的人口,是全世界主要的公共卫生问题。它被麸质衍生的肽所触发,这些肽具有异常高的脯氨酸-谷氨酰胺基序含量,并且对胃肠道消化酶的蛋白水解具有高度抵抗力。腹腔疾病的唯一治疗方法是严格,终生坚持无麸质饮食,这是有效的,但成本高且难以维持。因此,迫切需要用于乳糜泻的新型非饮食疗法。面筋降解酶是有前途的非饮食疗法,一些酶已在临床前或临床研究中进行了研究。荚膜鞘氨醇单胞菌脯氨酰内肽酶的组合(SC PEP)和谷氨酰胺特异性内切蛋白酶(大麦的EP-B2)称为latiglutenase在II期临床试验中显示出不足的益处,可能是由于其在胃环境中的酶活性较低。因此,提高酶活性对于SC PEP的临床应用至关重要。可以使用计算机辅助的合理蛋白质设计工具来增强酶的活性。在这项研究中,我们结合分子对接和分子动力学模拟来合理设计SC PEP突变体,并通过实验评估其活性。我们发现突变体的比活增加了90-103%,催化速率增加了80-202%。我们已经研究了这些突变体增强活性的潜在机制,并且发现SC PEP的β-螺旋结构域和催化结构域的构象转变对于酶活性是重要的,并且该转变受催化结构域和结构域界面中的残基影响;基材Pro和氧阴离子孔之间更短的距离对于提高SC PEP催化活性也至关重要。我们的结果为高活性SC PEP的合理设计提供了有用的信息,以加速乳糜泻的酶治疗候选药物的开发。










































 京公网安备 11010802027423号
京公网安备 11010802027423号