Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.cej.2020.126176 Yunqi Wang , Peng Zhou , Qingguo Wang , Xiaowei Huo , Xue Huang , Qian Ye , Hao Xu , Guanyu Zhou , Yuhang Liu , Jing Zhang
|
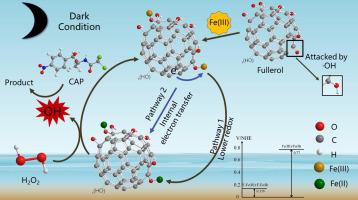
Hydroxylated fullerene, Fullerol, has applied in many areas because of its high electron affinity and antioxidant potential, while it was few used in wastewater treatment. In this study, the mechanism involved that Fullerol accelerated the regeneration of Fe(II) in the Fe(III)/H2O2 system under dark condition, which was investigated with chloramphenicol (CAP) as a model pollutant. In Fullerol/Fe(III)/H2O2 system, CAP removal was completely achieved at 60 min via a non-photochemical pathway. In addition, the quenching experiments and electron spin resonance (ESR) results indicated that hydroxyl radical (OH) was the main reactive oxygen species (ROSs). The Fullerol/Fe(III)/H2O2 system had a rate constant of CAP degradation about 5.0 × 10−2 min−1, which was about 22 times higher than it in Fe(III)/H2O2 Fenton-like system (2.3 × 10−3 min−1). Fe(II) was ascertained the effective component that was responsible for H2O2 activation to produce
OH, which derived from 1) the first pathway: Fullerol complexed with Fe(III) and the redox potential of the complex is much lower than Fe(III)/Fe(II); 2) the second pathway: Fullerol acted as an electron donor to reduce Fe(III) into Fe(II). Moreover, CAP degradation intermediates were identified and its degradation pathways were proposed. This study suggested a new approach for improving the Fe(III)/H2O2 system by accelerating Fe(III)/Fe(II) cycles with Fullerol.
中文翻译:

富勒醇通过非光化学途径加速Fe(III)/ Fe(II)循环,从而促进Fe(III)/ H 2 O 2系统中氯霉素的降解
羟基富勒烯,富勒醇由于其高的电子亲和力和抗氧化能力而已在许多领域得到应用,而很少用于废水处理。在这项研究中,机制涉及富勒醇在黑暗条件下促进Fe(III)/ H 2 O 2系统中Fe(II)的再生,并以氯霉素(CAP)作为模型污染物进行了研究。在Fullerol / Fe(III)/ H 2 O 2系统中,CAP的去除是在60分钟时通过非光化学途径完成的。此外,淬灭实验和电子自旋共振(ESR)结果表明,羟基自由基(OH)是主要的活性氧(ROSs)。富勒醇/ Fe(III)/ H 2 O 2系统的CAP降解速率常数为5.0×10 -2 min -1,是Fe(III)/ H 2 O 2类Fenton系统(2.3×10 -3 min -1)的约22倍。。确定了Fe(II)的有效成分,该成分负责激活H 2 O 2以产生
OH,源于1)第一条途径:富勒醇与Fe(III)络合,并且该络合物的氧化还原电势远低于Fe(III)/ Fe(II);2)第二种途径:富勒醇作为电子供体将Fe(III)还原为Fe(II)。此外,鉴定了CAP降解中间体,并提出了其降解途径。这项研究提出了一种新的方法,通过用富勒醇加速Fe(III)/ Fe(II)循环来改善Fe(III)/ H 2 O 2系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号