Cell Reports ( IF 7.5 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.celrep.2020.107873 Yan Qu 1 , Ji Wen 1 , Graham Thomas 1 , Wenjing Yang 1 , Weiwei Prior 1 , Wenqian He 1 , Purnima Sundar 1 , Xiao Wang 1 , Shobha Potluri 1 , Shahram Salek-Ardakani 1
|
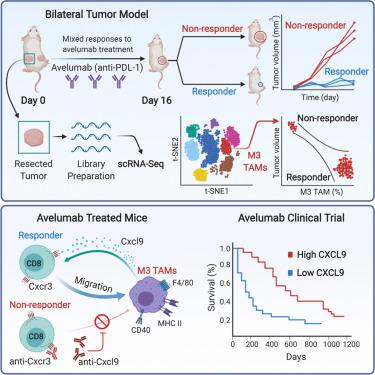
The tumor microenvironment is rich with immune-suppressive macrophages that are associated with cancer progression and resistance to immune checkpoint therapy. Using pre-treatment tumor biopsies complemented with single-cell RNA sequencing (RNA-seq), we characterize intratumoral immune heterogeneity to unveil potential mechanisms of resistance to avelumab (anti-PD-L1). We identify a proinflammatory F480+MHCII+Ly6Clo macrophage population that is associated with response rather than resistance to avelumab. These macrophages are the primary source of the interferon-inducible chemokine Cxcl9, which facilitates the recruitment of protective Cxcr3+ T cells. Consequently, the efficacy of avelumab in mouse tumor models is dependent on Cxcr3 and Cxcl9, and baseline levels of Cxcl9 in patients treated with avelumab are associated with clinical response and overall survival. These data suggest that, within the broadly immune-suppressive macrophage compartment, a pro-inflammatory population exists that promotes responsiveness to PD-L1 blockade.
中文翻译:

炎性表达Cxcl9的肿瘤相关巨噬细胞的基线频率预测对Avelumab治疗的反应。
肿瘤微环境富含免疫抑制巨噬细胞,这些巨噬细胞与癌症进展和对免疫检查点疗法的抵抗力有关。使用预处理的肿瘤活检与单细胞RNA测序(RNA-seq)互补,我们表征了肿瘤内免疫异质性,揭示了对avelumab(抗PD-L1)耐药的潜在机制。我们确定了促炎性F480 + MHCII + Ly6C lo巨噬细胞群与响应而不是对avelumab的耐药性相关。这些巨噬细胞是干扰素诱导型趋化因子Cxcl9的主要来源,可促进募集保护性Cxcr3 +T细胞。因此,avelumab在小鼠肿瘤模型中的疗效取决于Cxcr3和Cxcl9,并且在接受avelumab治疗的患者中Cxcl9的基线水平与临床反应和总体生存率相关。这些数据表明,在广泛的免疫抑制巨噬细胞区室中,存在促炎人群,其促进对PD-L1阻断的反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号