Cell Reports ( IF 7.5 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.celrep.2020.107864 Masaaki Sato 1 , Kotaro Mizuta 2 , Tanvir Islam 3 , Masako Kawano 4 , Yukiko Sekine 3 , Takashi Takekawa 5 , Daniel Gomez-Dominguez 6 , Alexander Schmidt 7 , Fred Wolf 8 , Karam Kim 4 , Hiroshi Yamakawa 9 , Masamichi Ohkura 10 , Min Goo Lee 11 , Tomoki Fukai 12 , Junichi Nakai 10 , Yasunori Hayashi 13
|
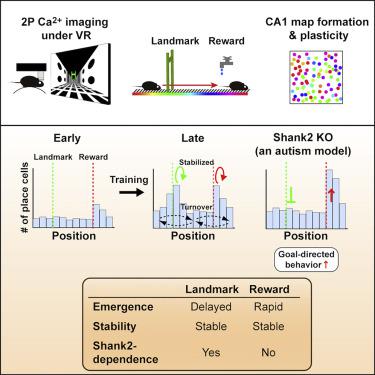
In the hippocampus, locations associated with salient features are represented by a disproportionately large number of neurons, but the cellular and molecular mechanisms underlying this over-representation remain elusive. Using longitudinal calcium imaging in mice learning to navigate in virtual reality, we find that the over-representation of reward and landmark locations are mediated by persistent and separable subsets of neurons, with distinct time courses of emergence and differing underlying molecular mechanisms. Strikingly, we find that in mice lacking Shank2, an autism spectrum disorder (ASD)-linked gene encoding an excitatory postsynaptic scaffold protein, the learning-induced over-representation of landmarks was absent whereas the over-representation of rewards was substantially increased, as was goal-directed behavior. These findings demonstrate that multiple hippocampal coding processes for unique types of salient features are distinguished by a Shank2-dependent mechanism and suggest that abnormally distorted hippocampal salience mapping may underlie cognitive and behavioral abnormalities in a subset of ASDs.
中文翻译:

海马中地标和奖励过多代表的独特机制。
在海马体中,与显着特征相关的位置由数量不成比例的大量神经元表示,但这种过度表达背后的细胞和分子机制仍然难以捉摸。在学习如何在虚拟现实中导航的小鼠中使用纵向钙成像,我们发现奖赏和地标位置的过度代表是由持久且可分离的神经元子集介导的,具有不同的出现时间过程和不同的潜在分子机制。令人惊讶的是,我们发现在缺少Shank2的小鼠中,一个自闭症谱系障碍(ASD)关联的编码兴奋性突触后支架蛋白的基因,没有学习引起的标志物的过度代表,而奖励的过度代表则大大增加了,如针对目标的行为。这些发现表明,不同类型的显着特征的多种海马编码过程是由Shank2依赖性机制来区分的,并表明异常扭曲的海马显着性图谱可能是ASD子集中认知和行为异常的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号