Cell Reports ( IF 7.5 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.celrep.2020.107858 Stephen Gray 1 , Emerson R Santiago 2 , Joshua S Chappie 2 , Paula E Cohen 1
|
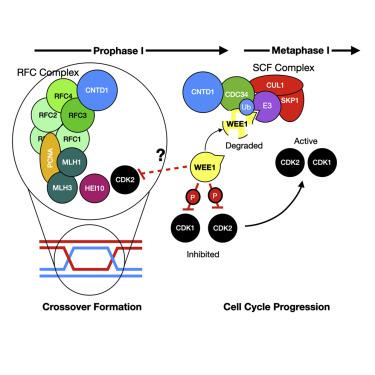
During mammalian meiotic prophase I, programmed DNA double-strand breaks are repaired by non-crossover or crossover events, the latter predominantly occurring via the class I crossover pathway and requiring the cyclin N-terminal domain-containing 1(CNTD1) protein. Using an epitope-tagged Cntd1 allele, we detect a short isoform of CNTD1 in vivo that lacks a predicted N-terminal cyclin domain and does not bind cyclin-dependent kinases. Instead, we find that the short-form CNTD1 variant associates with components of the replication factor C (RFC) machinery to facilitate crossover formation, and with the E2 ubiquitin conjugating enzyme, CDC34, to regulate ubiquitylation and subsequent degradation of the WEE1 kinase, thereby modulating cell-cycle progression. We propose that these interactions facilitate a role for CNTD1 as a stop-go regulator during prophase I, ensuring accurate and complete crossover formation before allowing metaphase progression and the first meiotic division.
中文翻译:

细胞周期蛋白N端域包含1协调减数分裂交叉形成与细胞周期独立于细胞周期蛋白的方式。
在哺乳动物减数分裂前期I期间,非交叉或交叉事件可修复程序化的DNA双链断裂,后者主要通过I类交叉途径发生,并需要细胞周期蛋白N末端域含1(CNTD1)蛋白。使用带有表位标签的Cntd1等位基因,我们在体内检测到CNTD1的短型缺少预测的N末端细胞周期蛋白结构域,并且不结合细胞周期蛋白依赖性激酶。相反,我们发现简短的CNTD1变体与复制因子C(RFC)机制的组成部分相关,以促进交叉形成,并与E2泛素结合酶CDC34一起调节WEE1激酶的泛素化和随后的降解,从而调节细胞周期进程。我们建议这些相互作用促进CNTD1在前期I期间起停停调节剂的作用,确保在允许中期进展和第一次减数分裂之前确保准确和完整的交叉形成。









































 京公网安备 11010802027423号
京公网安备 11010802027423号