Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.bioorg.2020.104072 Gabriele Murineddu 1 , Battistina Asproni 1 , Paola Corona 1 , Stefania Gessi 2 , Stefania Merighi 2 , Enrica Battistello 2 , Chiara Sturaro 3 , Girolamo Calò 3 , Nicoletta Galeotti 4 , Veronika Temml 5 , Sonja Herdlinger 6 , Daniela Schuster 7 , Gerard A Pinna 1
|
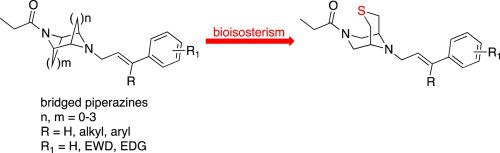
A small library of 3-thia-7,9-diazabicyclo[3.3.1]nonanes was synthesized and their opioid receptors affinity and selectivity evaluated. Among these novel sulfur-bridged compounds, the (E) 9-[3′-(3-chlorophenyl)-but-2′-en-1′-yl]-7-propionyl-3-thia-7,9-diazabicyclo[3.3.1]nonane 2i emerged as the derivative with the highest μ receptor affinity (Ki = 85 nM) and selectivity (Ki μ/δ = 58.8, Ki μ/κ > 117.6). The antinociceptive activity of 2i was also evaluated in acute thermal pain. Docking studies disclosed the specific pattern of interactions of these derivatives.
中文翻译:

一类新型的硫桥二氮杂双环[3.3.1]壬烷的合成,生物学评价和对接研究。
合成了3-thia-7,9-diazabicyclo [3.3.1]壬烷的小文库,并评估了其阿片受体的亲和力和选择性。在这些新颖的硫桥化合物中,(E)9- [3'-(3-氯苯基)-but-2'-en-1'-基] -7-丙酰基-3-thia-7,9-二氮杂双环[3.3.1]壬烷2I成为该衍生物具有最高μ受体的亲和力(ķ我 = 85 nM)的和选择性(ķ我μ/ δ = 58.8,ķ我μ/κ> 117.6)。在急性热痛中还评估了2i的抗伤害感受活性。对接研究揭示了这些衍生物相互作用的具体模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号