Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.abb.2020.108442 Revansiddha H. Katte , Ruey-Hwang Chou , Chin Yu
|
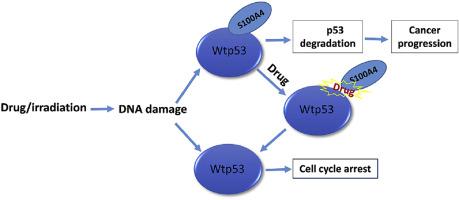
Metastasis-associated S100A4 protein is a small calcium-binding protein typically overexpressed in several tumor forms, and it is widely accepted that S100A4 plays a significant role in the metastasis of cancer. Tumor suppressor p53 is one of the S100A4's main targets. Previous reports show that through p53, S100A4 regulates collagen expression and cell proliferation. When S100A4 interacts with p53, the S100A4 destabilizes wild type p53. In the current study, based on 1H–15N HSQC NMR experiments and HADDOCK results, S100A4 interacts with the intrinsically unstructured transactivation domain (TAD) of the protein p53 and the pentamidine molecules in the presence of calcium ions. Our results suggest that the p53 TAD and pentamidine molecules share similar binding sites on the S100A4 protein. This observation indicates that a competitive binding mechanism can interfere with the binding of S100A4-p53 and increase the level of p53. Also, we compare different aspects of p53 activity in the WST-1 test using MCF 7 cells. We found that the presence of a pentamidine molecule results in higher p53 activity, which is also reflected in less cell proliferation. Collectively, our results indicate that disrupting the S100A4-p53 interaction would prevent cancer progression, and thus S100A4-p53 inhibitors provide a new avenue for cancer therapy.
中文翻译:

喷他idine抑制S100A4-p53相互作用并降低细胞增殖活性。
转移相关的S100A4蛋白是一种钙结合蛋白,通常在几种肿瘤形式中过表达,并且被广泛接受,S100A4在癌症的转移中起重要作用。肿瘤抑制因子p53是S100A4的主要目标之一。先前的报道表明,通过p53,S100A4调节胶原蛋白的表达和细胞增殖。当S100A4与p53相互作用时,S100A4使野生型p53不稳定。在本研究中,基于1 H– 15N HSQC NMR实验和HADDOCK结果表明,在钙离子存在下,S100A4与蛋白质p53和喷他tam分子的固有非结构化反式激活结构域(TAD)相互作用。我们的结果表明,p53 TAD和喷他idine分子在S100A4蛋白上具有相似的结合位点。该观察结果表明竞争性结合机制可干扰S100A4-p53的结合并增加p53的水平。此外,我们在使用MCF 7细胞的WST-1测试中比较了p53活性的不同方面。我们发现,喷他idine分子的存在导致较高的p53活性,这也反映在较少的细胞增殖中。总体而言,我们的结果表明,破坏S100A4-p53相互作用将阻止癌症的发展,











































 京公网安备 11010802027423号
京公网安备 11010802027423号