当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mitochondria-targeted nanospheres with deep tumor penetration for photo/starvation therapy.
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020-07-06 , DOI: 10.1039/d0tb00001a Yong Wang 1 , Bo Wang , Liang Zhang , Ju Huang , Pan Li , Yahui Zhao , Chen Zhou , Mei Liu , Weiwei Li , Jie He
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020-07-06 , DOI: 10.1039/d0tb00001a Yong Wang 1 , Bo Wang , Liang Zhang , Ju Huang , Pan Li , Yahui Zhao , Chen Zhou , Mei Liu , Weiwei Li , Jie He
Affiliation
|
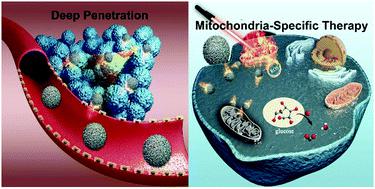
Tumor masses are three-dimensional (3D). The abnormal physiology of solid tumors is a great barrier to anticancer drug delivery, and the development of effective therapeutic strategies for cancer treatment remains highly challenging. In this study, we have rationally designed IR780 and glucose oxidase (GOx) based poly lactic-co-glycolic acid (PLGA) nanospheres, which can not only selectively accumulate in mitochondria, but also penetrate into 3D tumors deeply at the same time, achieving synergistic treatment of phototherapy and enzyme (GOx)-induced starvation therapy under dual-imaging guidance/monitoring. The lipophilic cationic properties of IR780 enable the nanospheres to penetrate into deep tumor tissues, which has been demonstrated by in vitro 3D tumor modeling and in vivo tumor reconstruction. Meanwhile, the inherent structure of IR780 endows the nanospheres with mitochondrial targeting capability. As mitochondria are susceptible to hyperpyrexia and reactive oxygen species (ROS), mitochondria-targeted phototherapy shows more efficient therapeutic performance. Furthermore, the starvation effect of GOx can cut off the nutrition supply to tumor cells, enhancing the energy metabolism disorder of tumor cells after mitochondrial damage induced by phototherapy, further increasing the damage to tumor cells. In addition, the therapeutic process can be guided/monitored by photoacoustic (PA) and fluorescence (FL) dual imaging. Due to the incorporation of multiple modalities, these nanospheres are promising for cancer theranostics.
中文翻译:

线粒体靶向纳米球具有深层的肿瘤渗透能力,可用于光/饥饿疗法。
肿瘤块是三维(3D)的。实体瘤的异常生理学是抗癌药物递送的巨大障碍,并且开发有效的癌症治疗方法仍然具有很高的挑战性。在这项研究中,我们已经设计合理IR780和基于聚乳酸-葡萄糖氧化酶(GOx)共同-glycolic乙酸(PLGA)纳米球,它不仅可以在同一时间在线粒体中积累选择性,而且还渗透到3D肿瘤的深入,实现在双成像引导/监测下对光疗和酶(GOx)诱导的饥饿疗法进行协同治疗。IR780的亲脂阳离子特性使纳米球能够渗透到深部肿瘤组织中,这已通过体外3D肿瘤建模和体内肿瘤重建。同时,IR780的固有结构使纳米球具有线粒体靶向能力。由于线粒体易受高热和活性氧(ROS)的影响,针对线粒体的光疗显示出更有效的治疗性能。此外,GOx的饥饿作用可以切断对肿瘤细胞的营养供应,增强了光疗引起的线粒体损伤后肿瘤细胞的能量代谢紊乱,进一步增加了对肿瘤细胞的损伤。此外,治疗过程可以通过光声(PA)和荧光(FL)双重成像进行引导/监控。由于多种形式的结合,这些纳米球有望用于癌症治疗。
更新日期:2020-09-02
中文翻译:

线粒体靶向纳米球具有深层的肿瘤渗透能力,可用于光/饥饿疗法。
肿瘤块是三维(3D)的。实体瘤的异常生理学是抗癌药物递送的巨大障碍,并且开发有效的癌症治疗方法仍然具有很高的挑战性。在这项研究中,我们已经设计合理IR780和基于聚乳酸-葡萄糖氧化酶(GOx)共同-glycolic乙酸(PLGA)纳米球,它不仅可以在同一时间在线粒体中积累选择性,而且还渗透到3D肿瘤的深入,实现在双成像引导/监测下对光疗和酶(GOx)诱导的饥饿疗法进行协同治疗。IR780的亲脂阳离子特性使纳米球能够渗透到深部肿瘤组织中,这已通过体外3D肿瘤建模和体内肿瘤重建。同时,IR780的固有结构使纳米球具有线粒体靶向能力。由于线粒体易受高热和活性氧(ROS)的影响,针对线粒体的光疗显示出更有效的治疗性能。此外,GOx的饥饿作用可以切断对肿瘤细胞的营养供应,增强了光疗引起的线粒体损伤后肿瘤细胞的能量代谢紊乱,进一步增加了对肿瘤细胞的损伤。此外,治疗过程可以通过光声(PA)和荧光(FL)双重成像进行引导/监控。由于多种形式的结合,这些纳米球有望用于癌症治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号