当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface precipitation of Mn2+ on clay minerals enhances Cd2+ sorption under anoxic conditions.
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-07-06 , DOI: 10.1039/d0em00155d Natacha Van Groeningen 1 , Blanche Glück , Iso Christl , Ruben Kretzschmar
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-07-06 , DOI: 10.1039/d0em00155d Natacha Van Groeningen 1 , Blanche Glück , Iso Christl , Ruben Kretzschmar
Affiliation
|
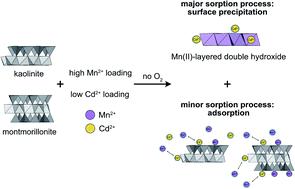
The influence of Mn2+ on the sorption of metal(loid)s onto clay minerals is still unclear despite its relevance in suboxic and anoxic environments which often exhibit elevated dissolved Mn2+ concentrations. In this study, the effects of Mn2+ on Cd2+ sorption to two types of clay minerals, a well-crystalline natural kaolinite (KGa-1b) and a synthetic montmorillonite (Syn-1), were investigated. Batch experiments on Mn2+ and Cd2+ sorption to Ca-saturated KGa-1b and Syn-1 were conducted under anoxic conditions. At low Mn2+ and Cd2+ concentrations (1 and 5 µM), both metals exhibited similar affinity for sorption to the clays, suggesting that elevated Mn2+ concentrations might effectively decrease Cd2+ sorption as predicted using a three-plane surface complexation model. However, competitive Mn–Cd experiments at higher concentrations (≥50 µM) revealed that for both clay minerals, the presence of Mn2+ increased Cd2+ sorption to the solid phases. Although solutions were undersaturated with respect to known Mn(II) solid phases, analysis using X-ray absorption spectroscopy (XAS) evidenced the formation of Mn(II)-containing solid phases which can specifically adsorb or incorporate Cd2+. This process, which was mediated by the presence of clay minerals, overcompensated the decrease in Cd2+ adsorption to clay surfaces due to competition with Mn2+. We conclude that, contrary to predictions based on a competitive surface complexation model, elevated Mn2+ concentrations can contribute to decrease dissolved Cd2+ concentrations in anoxic clay-containing environments, such as contaminated sediments or flooded paddy soils.
中文翻译:

在缺氧条件下,Mn2 +在粘土矿物上的表面沉淀会增强Cd2 +的吸附。
尽管Mn 2+在通常表现出较高的溶解Mn 2+浓度的低氧和缺氧环境中具有相关性,但对金属(胶体)在粘土矿物上的吸附的影响仍然不清楚。在这项研究中,研究了Mn 2+对Cd 2+吸附到两种类型的粘土矿物上的影响,这两种晶体是高结晶度的天然高岭石(KGa-1b)和合成的蒙脱石(Syn-1)。在缺氧条件下进行了对Mn 2+和Cd 2+吸附到Ca饱和的KGa-1b和Syn-1的分批实验。Mn 2+和Cd 2+低时浓度(分别为1和5 µM),两种金属对粘土的吸附均表现出相似的亲和力,这表明,如使用三平面表面络合模型所预测的,升高的Mn 2+浓度可能有效降低Cd 2+的吸附。但是,在较高浓度(≥50µM)下的竞争性Mn–Cd实验表明,对于两种粘土矿物,Mn 2+的存在都会增加Cd 2+对固相的吸附。尽管溶液相对于已知的Mn(II)固相而言不饱和,但使用X射线吸收光谱法(XAS)进行的分析表明,形成了可以特异性吸附或掺入Cd 2+的含Mn(II)固相。。该过程是由粘土矿物的存在介导的,该过程过度补偿了由于与Mn 2+的竞争而使Cd 2+对粘土表面的吸附减少。我们得出结论,与基于竞争性表面络合模型的预测相反,升高的Mn 2+浓度可有助于降低含氧粘土环境中的溶解Cd 2+浓度,例如受污染的沉积物或淹没的稻田土壤。
更新日期:2020-08-19
中文翻译:

在缺氧条件下,Mn2 +在粘土矿物上的表面沉淀会增强Cd2 +的吸附。
尽管Mn 2+在通常表现出较高的溶解Mn 2+浓度的低氧和缺氧环境中具有相关性,但对金属(胶体)在粘土矿物上的吸附的影响仍然不清楚。在这项研究中,研究了Mn 2+对Cd 2+吸附到两种类型的粘土矿物上的影响,这两种晶体是高结晶度的天然高岭石(KGa-1b)和合成的蒙脱石(Syn-1)。在缺氧条件下进行了对Mn 2+和Cd 2+吸附到Ca饱和的KGa-1b和Syn-1的分批实验。Mn 2+和Cd 2+低时浓度(分别为1和5 µM),两种金属对粘土的吸附均表现出相似的亲和力,这表明,如使用三平面表面络合模型所预测的,升高的Mn 2+浓度可能有效降低Cd 2+的吸附。但是,在较高浓度(≥50µM)下的竞争性Mn–Cd实验表明,对于两种粘土矿物,Mn 2+的存在都会增加Cd 2+对固相的吸附。尽管溶液相对于已知的Mn(II)固相而言不饱和,但使用X射线吸收光谱法(XAS)进行的分析表明,形成了可以特异性吸附或掺入Cd 2+的含Mn(II)固相。。该过程是由粘土矿物的存在介导的,该过程过度补偿了由于与Mn 2+的竞争而使Cd 2+对粘土表面的吸附减少。我们得出结论,与基于竞争性表面络合模型的预测相反,升高的Mn 2+浓度可有助于降低含氧粘土环境中的溶解Cd 2+浓度,例如受污染的沉积物或淹没的稻田土壤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号