当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
cine-Silylative Ring-Opening of α-Methyl Azacycles Enabled by the Silylium-Induced C-N Bond Cleavage
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-06 , DOI: 10.1021/jacs.0c05241 Jianbo Zhang 1, 2 , Sukbok Chang 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-06 , DOI: 10.1021/jacs.0c05241 Jianbo Zhang 1, 2 , Sukbok Chang 1, 2
Affiliation
|
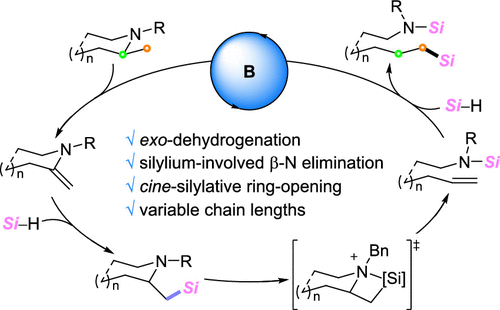
Described herein is the development of a borane-catalyzed cine-silylative ring-opening of α-methyl azacycles. This transformation involves four-step cascade processes: (i) exo-dehydrogenation of alicyclic amine, (ii) hydrosilylation of resultant enamine, (iii) silylium-induced cis-β-amino elimination to open the ring skeleton, and (iv) hydrosilylation of terminal olefin. The present borane catalysis also works efficiently for the C-N bond cleavage of acyclic tertiary amines. On the basis of experimental and computational studies, the silicon atom was elucidated to play a pivotal role in the β-amino elimination step.
中文翻译:

由甲硅烷基诱导的 CN 键裂解实现的 α-甲基氮杂环的电影-甲硅烷基化开环
本文描述了硼烷催化的α-甲基氮杂环的电影-甲硅烷基化开环的开发。这种转化涉及四步级联过程:(i) 脂环胺的外脱氢,(ii) 所得烯胺的氢化硅烷化,(iii) 甲硅烷诱导的顺式-β-氨基消除以打开环骨架,以及 (iv) 氢化硅烷化端烯烃。本硼烷催化对于无环叔胺的 CN 键断裂也有效。在实验和计算研究的基础上,阐明了硅原子在 β-氨基消除步骤中起关键作用。
更新日期:2020-07-06
中文翻译:

由甲硅烷基诱导的 CN 键裂解实现的 α-甲基氮杂环的电影-甲硅烷基化开环
本文描述了硼烷催化的α-甲基氮杂环的电影-甲硅烷基化开环的开发。这种转化涉及四步级联过程:(i) 脂环胺的外脱氢,(ii) 所得烯胺的氢化硅烷化,(iii) 甲硅烷诱导的顺式-β-氨基消除以打开环骨架,以及 (iv) 氢化硅烷化端烯烃。本硼烷催化对于无环叔胺的 CN 键断裂也有效。在实验和计算研究的基础上,阐明了硅原子在 β-氨基消除步骤中起关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号