当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tartryl-CoA inhibits succinyl-CoA synthetase.
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-07-06 , DOI: 10.1107/s2053230x20008201 Ji Huang 1 , Marie E Fraser 1
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-07-06 , DOI: 10.1107/s2053230x20008201 Ji Huang 1 , Marie E Fraser 1
Affiliation
|
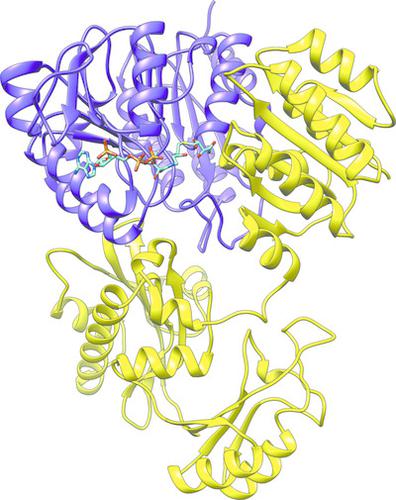
Succinyl‐CoA synthetase (SCS) catalyzes the only substrate‐level phosphorylation step in the tricarboxylic acid cycle. Human GTP‐specific SCS (GTPSCS), an αβ‐heterodimer, was produced in Escherichia coli. The purified protein crystallized from a solution containing tartrate, CoA and magnesium chloride, and a crystal diffracted to 1.52 Å resolution. Tartryl‐CoA was discovered to be bound to GTPSCS. The CoA portion lies in the amino‐terminal domain of the α‐subunit and the tartryl end extends towards the catalytic histidine residue. The terminal carboxylate binds to the phosphate‐binding site of GTPSCS.
中文翻译:

酒石酰辅酶 A 抑制琥珀酰辅酶 A 合成酶。
琥珀酰辅酶A合成酶 (SCS) 催化三羧酸循环中唯一的底物水平磷酸化步骤。人 GTP 特异性 SCS (GTPSCS) 是一种 αβ-异二聚体,由大肠杆菌产生。纯化的蛋白质从含有酒石酸盐、CoA 和氯化镁的溶液中结晶,晶体衍射至 1.52 Å 分辨率。发现 Tartryl-CoA 与 GTPSCS 结合。 CoA 部分位于 α 亚基的氨基末端结构域,酒石酰基末端向催化组氨酸残基延伸。末端羧酸盐与 GTPSCS 的磷酸盐结合位点结合。
更新日期:2020-07-06
中文翻译:

酒石酰辅酶 A 抑制琥珀酰辅酶 A 合成酶。
琥珀酰辅酶A合成酶 (SCS) 催化三羧酸循环中唯一的底物水平磷酸化步骤。人 GTP 特异性 SCS (GTPSCS) 是一种 αβ-异二聚体,由大肠杆菌产生。纯化的蛋白质从含有酒石酸盐、CoA 和氯化镁的溶液中结晶,晶体衍射至 1.52 Å 分辨率。发现 Tartryl-CoA 与 GTPSCS 结合。 CoA 部分位于 α 亚基的氨基末端结构域,酒石酰基末端向催化组氨酸残基延伸。末端羧酸盐与 GTPSCS 的磷酸盐结合位点结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号