当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and biochemical characterization of novel carbonic anhydrases from Phaeodactylum tricornutum.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-07-06 , DOI: 10.1107/s2059798320007202 Shengyang Jin 1 , Daniela Vullo 2 , Silvia Bua 2 , Alessio Nocentini 2 , Claudiu T Supuran 2 , Yong Gui Gao 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-07-06 , DOI: 10.1107/s2059798320007202 Shengyang Jin 1 , Daniela Vullo 2 , Silvia Bua 2 , Alessio Nocentini 2 , Claudiu T Supuran 2 , Yong Gui Gao 1
Affiliation
|
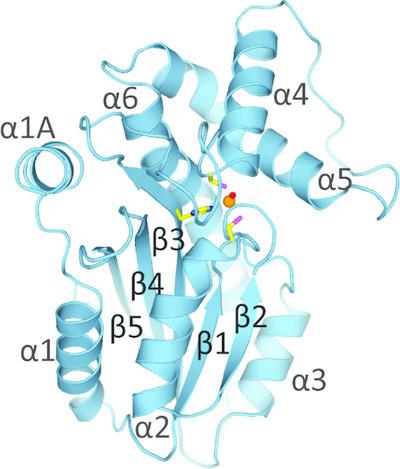
Carbonic anhydrases (CAs) are a well characterized family of metalloenzymes that are highly efficient in facilitating the interconversion between carbon dioxide and bicarbonate. Recently, CA activity has been associated with the LCIB (limiting CO2‐inducible protein B) protein family, which has been an interesting target in aquatic photosynthetic microorganisms. To gain further insight into the catalytic mechanism of this new group of CAs, the X‐ray structure of a highly active LCIB homolog (PtLCIB3) from the diatom Phaeodactylum tricornutum was determined. The CA activities of PtLCIB3, its paralog PtLCIB4 and a variety of their mutants were also measured. It was discovered that PtLCIB3 has a classic β‐CA fold and its overall structure is highly similar to that of its homolog PtLCIB4. Subtle structural alterations between PtLCIB3 and PtLCIB4 indicate that an alternative proton‐shuttle cavity could perhaps be one reason for their remarkable difference in CA activity. A potential alternative proton‐shuttle route in the LCIB protein family is suggested based on these results.
中文翻译:

结构和生化表征的新型碳酸酐酶的三角果。
碳酸酐酶(CAs)是金属酶的一个特征鲜明的家族,在促进二氧化碳和碳酸氢根之间的相互转化方面非常高效。最近,CA活性已经与LCIB(限制CO 2诱导的蛋白B)蛋白家族相关联,这是水生光合微生物中一个有趣的目标。为了进一步了解这组新的CA的催化机理,研究了来自硅藻Phaeodactylum tricornutum的高活性LCIB同系物(PtLCIB3)的X射线结构。被确定。还测量了PtLCIB3,其旁系同源物PtLCIB4及其各种突变体的CA活性。已发现PtLCIB3具有经典的β-CA折叠,其整体结构与其同源PtLCIB4高度相似。PtLCIB3和PtLCIB4之间的细微结构变化表明,替代质子-穿梭腔可能是其CA活性显着不同的原因之一。根据这些结果,暗示了LCIB蛋白家族中潜在的质子穿梭途径。
更新日期:2020-07-06
中文翻译:

结构和生化表征的新型碳酸酐酶的三角果。
碳酸酐酶(CAs)是金属酶的一个特征鲜明的家族,在促进二氧化碳和碳酸氢根之间的相互转化方面非常高效。最近,CA活性已经与LCIB(限制CO 2诱导的蛋白B)蛋白家族相关联,这是水生光合微生物中一个有趣的目标。为了进一步了解这组新的CA的催化机理,研究了来自硅藻Phaeodactylum tricornutum的高活性LCIB同系物(PtLCIB3)的X射线结构。被确定。还测量了PtLCIB3,其旁系同源物PtLCIB4及其各种突变体的CA活性。已发现PtLCIB3具有经典的β-CA折叠,其整体结构与其同源PtLCIB4高度相似。PtLCIB3和PtLCIB4之间的细微结构变化表明,替代质子-穿梭腔可能是其CA活性显着不同的原因之一。根据这些结果,暗示了LCIB蛋白家族中潜在的质子穿梭途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号