当前位置:
X-MOL 学术
›
J. Neurosci. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Delta opioid receptor activation modulates affective pain and modality-specific pain hypersensitivity associated with chronic neuropathic pain
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2020-07-05 , DOI: 10.1002/jnr.24680 Catherine M Cahill 1 , Sarah V Holdridge 2 , Shiwei Steve Liu 1 , Lihua Xue 2 , Claire Magnussen 2 , Edmund Ong 2 , Patrick Grenier 2 , Anne Sutherland 2 , Mary C Olmstead 3
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2020-07-05 , DOI: 10.1002/jnr.24680 Catherine M Cahill 1 , Sarah V Holdridge 2 , Shiwei Steve Liu 1 , Lihua Xue 2 , Claire Magnussen 2 , Edmund Ong 2 , Patrick Grenier 2 , Anne Sutherland 2 , Mary C Olmstead 3
Affiliation
|
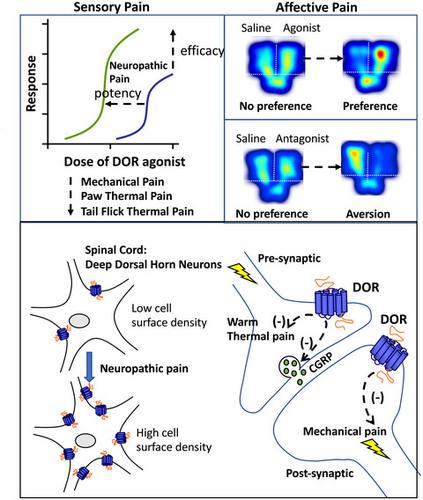
Delta opioid receptor (DOR) agonists alleviate nociceptive behaviors in various chronic pain models, including neuropathic pain, while having minimal effect on sensory thresholds in the absence of injury. The mechanisms underlying nerve injury-induced enhancement of DOR function are unclear. We used a peripheral nerve injury (PNI) model of neuropathic pain to assess changes in the function and localization of DORs in mice and rats. Intrathecal administration of DOR agonists reversed mechanical allodynia and thermal hyperalgesia. The dose-dependent thermal antinociceptive effects of DOR agonists were shifted to the left in PNI rats. Administration of DOR agonists produced a conditioned place preference in PNI, but not in sham, animals, whereas the DOR antagonist naltrindole produced a place aversion in PNI, but not in sham, mice, suggesting the engagement of endogenous DOR activity in suppressing pain associated with the injury. GTPγS autoradiography revealed an increase in DOR function in the dorsal spinal cord, ipsilateral to PNI. Immunogold electron microscopy and in vivo fluorescent agonist assays were used to assess changes in the ultrastructural localization of DORs in the spinal dorsal horn. In shams, DORs were primarily localized within intracellular compartments. PNI significantly increased the cell surface expression of DORs within lamina IV-V dendritic profiles. Using neonatal capsaicin treatment, we identified that DOR agonist-induced thermal antinociception was mediated via receptors expressed on primary afferent sensory neurons but did not alter mechanical thresholds. These data reveal that the regulation of DORs following PNI and suggest the importance of endogenous activation of DORs in regulating chronic pain states.
中文翻译:

三角洲阿片受体激活调节与慢性神经性疼痛相关的情感疼痛和方式特异性疼痛超敏反应
δ 阿片受体 (DOR) 激动剂可减轻各种慢性疼痛模型(包括神经性疼痛)中的伤害感受行为,同时在没有受伤的情况下对感觉阈值的影响最小。神经损伤诱导 DOR 功能增强的机制尚不清楚。我们使用神经性疼痛的周围神经损伤 (PNI) 模型来评估 DOR 在小鼠和大鼠中的功能和定位变化。DOR 激动剂的鞘内给药可逆转机械异常性疼痛和热痛觉过敏。DOR 激动剂的剂量依赖性热镇痛作用在 PNI 大鼠中向左移动。DOR 激动剂的给药在 PNI 中产生了条件性位置偏好,但在假动物中没有,而 DOR 拮抗剂纳曲多在 PNI 中产生了位置厌恶,但在假小鼠中没有,表明内源性 DOR 活动参与抑制与损伤相关的疼痛。GTPγS 放射自显影显示 PNI 同侧的背侧脊髓 DOR 功能增加。免疫金电子显微镜和体内荧光激动剂测定用于评估脊髓背角中 DOR 超微结构定位的变化。在假手术中,DORs 主要位于细胞内隔间内。PNI 显着增加了 IV-V 层树突状结构中 DOR 的细胞表面表达。使用新生儿辣椒素治疗,我们发现 DOR 激动剂诱导的热镇痛作用是通过初级传入感觉神经元上表达的受体介导的,但没有改变机械阈值。这些数据揭示了 PNI 后 DOR 的调节,并表明 DOR 内源性激活在调节慢性疼痛状态中的重要性。
更新日期:2020-07-05
中文翻译:

三角洲阿片受体激活调节与慢性神经性疼痛相关的情感疼痛和方式特异性疼痛超敏反应
δ 阿片受体 (DOR) 激动剂可减轻各种慢性疼痛模型(包括神经性疼痛)中的伤害感受行为,同时在没有受伤的情况下对感觉阈值的影响最小。神经损伤诱导 DOR 功能增强的机制尚不清楚。我们使用神经性疼痛的周围神经损伤 (PNI) 模型来评估 DOR 在小鼠和大鼠中的功能和定位变化。DOR 激动剂的鞘内给药可逆转机械异常性疼痛和热痛觉过敏。DOR 激动剂的剂量依赖性热镇痛作用在 PNI 大鼠中向左移动。DOR 激动剂的给药在 PNI 中产生了条件性位置偏好,但在假动物中没有,而 DOR 拮抗剂纳曲多在 PNI 中产生了位置厌恶,但在假小鼠中没有,表明内源性 DOR 活动参与抑制与损伤相关的疼痛。GTPγS 放射自显影显示 PNI 同侧的背侧脊髓 DOR 功能增加。免疫金电子显微镜和体内荧光激动剂测定用于评估脊髓背角中 DOR 超微结构定位的变化。在假手术中,DORs 主要位于细胞内隔间内。PNI 显着增加了 IV-V 层树突状结构中 DOR 的细胞表面表达。使用新生儿辣椒素治疗,我们发现 DOR 激动剂诱导的热镇痛作用是通过初级传入感觉神经元上表达的受体介导的,但没有改变机械阈值。这些数据揭示了 PNI 后 DOR 的调节,并表明 DOR 内源性激活在调节慢性疼痛状态中的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号