当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphorus and Sulfur Co-Doped Cobaltous Oxide Synthesized by an Inorganic-Salt-Assisted Method: Reaction Mechanism and Electrocatalytic Application.
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-07-06 , DOI: 10.1002/cplu.202000306 Jagadish Chandra Roy 1 , Mohammad Al-Mamun 1 , Huajie Yin 1 , Yuhai Dou 1 , Lei Zhang 1, 2 , Porun Liu 1 , Yun Wang 1 , Yu Lin Zhong 1 , Huijun Zhao 1, 2
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-07-06 , DOI: 10.1002/cplu.202000306 Jagadish Chandra Roy 1 , Mohammad Al-Mamun 1 , Huajie Yin 1 , Yuhai Dou 1 , Lei Zhang 1, 2 , Porun Liu 1 , Yun Wang 1 , Yu Lin Zhong 1 , Huijun Zhao 1, 2
Affiliation
|
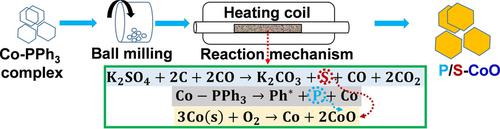
An inorganic‐salt‐assisted synthesis of non‐metallic heteroatom (phosphorus and sulfur) co‐doped cobaltous oxide (P/S‐CoO) has been reported. Potassium sulphate (K2SO4) was used as inorganic source of sulfur (S), while triphenyl phosphine (PPh3) was used as phosphorus (P) source. A stepwise mechanistic investigation into the doping process revealed that the decomposition of PPh3 triggered the release of both the elemental sulfur and phosphorus because of the reducing reaction environment. The transformation of cobalt‐PPh3 complex into cubic cobalt (II) oxide along with the successful co‐doping (P and S) was achieved by high temperature calcination at 800 °C but preserved the bulk CoO crystalline structure. The as synthesized P/S‐CoO demonstrated an unprecedented enhancement on the oxygen evolution activity compare to that of pristine CoO with the current density of 10 mA/cm2 at the overpotential of 293 mV in 1.0 M KOH electrolyte and profound stability at different current densities.
中文翻译:

无机盐辅助法合成的磷硫共掺杂氧化钴:反应机理及电催化应用。
据报道,无机盐辅助合成了非金属杂原子(磷和硫)共掺杂的氧化钴(P / S-CoO)。硫酸钾(K 2 SO 4)被用作无机硫(S),而三苯基膦(PPh 3)被用作磷(P)源。对掺杂过程的逐步机理研究表明,由于反应环境的还原,PPh 3的分解触发了元素硫和磷的释放。钴-PPh 3的转化通过在800°C的高温煅烧,可将其复杂地合成为立方氧化钴(II)和成功的共掺杂(P和S),但保留了整体的CoO晶体结构。所合成的P / S-CoO与原始CoO相比,在1.0 M KOH电解液中的293 mV超电势下的电流密度为10 mA / cm 2时,显示出比原始CoO更高的放氧活性,并且在不同电流下具有很强的稳定性密度。
更新日期:2020-07-30
中文翻译:

无机盐辅助法合成的磷硫共掺杂氧化钴:反应机理及电催化应用。
据报道,无机盐辅助合成了非金属杂原子(磷和硫)共掺杂的氧化钴(P / S-CoO)。硫酸钾(K 2 SO 4)被用作无机硫(S),而三苯基膦(PPh 3)被用作磷(P)源。对掺杂过程的逐步机理研究表明,由于反应环境的还原,PPh 3的分解触发了元素硫和磷的释放。钴-PPh 3的转化通过在800°C的高温煅烧,可将其复杂地合成为立方氧化钴(II)和成功的共掺杂(P和S),但保留了整体的CoO晶体结构。所合成的P / S-CoO与原始CoO相比,在1.0 M KOH电解液中的293 mV超电势下的电流密度为10 mA / cm 2时,显示出比原始CoO更高的放氧活性,并且在不同电流下具有很强的稳定性密度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号