当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold(I)-catalyzed intramolecular [4 + 2] cycloaddition of ortho-(N-tethered 1,6-diynyl)benzaldehydes to 3,4-dihydrobenzo[f]isoquinolin-1(2H)-ones
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.tetlet.2020.152200 Jiankai Zhong , Rui Hu , Jingjing Sang , Weidong Rao
中文翻译:

金(I)催化的邻-(N-束缚的1,6-6-二炔基)苯甲醛的分子内[4 + 2]环加成反应成3,4-二氢苯并[ f ]异喹啉-1(2H)-
更新日期:2020-07-15
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.tetlet.2020.152200 Jiankai Zhong , Rui Hu , Jingjing Sang , Weidong Rao
|
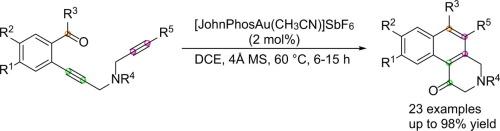
An efficient synthetic method to prepare 3,4-dihydrobenzo[f]isoquinolin-1(2H)-ones through gold(I)-catalyzed intramolecular [4 + 2] benzannulation of o-(N-tethered 1,6-diynyl)benzaldehydes at a low catalyst loading of 2 mol% is described. The product of 3,4-dihydrobenzo[f]isoquinolin-1(2H)-ones can be easily transformed to benzo[f]isoquinolin-1-ols by simply treating with phenylmagnesium bromide under mild conditions. The structures of compounds 2o and 2w were also ascertained by X-ray crystallographic analysis.
中文翻译:

金(I)催化的邻-(N-束缚的1,6-6-二炔基)苯甲醛的分子内[4 + 2]环加成反应成3,4-二氢苯并[ f ]异喹啉-1(2H)-
一种有效的合成方法,通过金(I)催化的邻-(N-拴系的1,6-二炔基)苯甲醛的分子内[4 + 2]苯环化制备3,4-二氢苯并[ f ]异喹啉-1(2H)-酮。描述了在2mol%的低催化剂载量下的催化剂。3,4-二氢苯并[ f ]异喹啉-1(2H)-one的产物可以通过在温和的条件下简单地用苯基溴化镁处理而轻易地转化为苯并[ f ]异喹啉-1-醇。化合物2o和2w的结构也通过X射线晶体学分析确定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号