Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.tetlet.2020.152213 Nao Umemoto , Ayumi Imayoshi , Kazunori Tsubaki
|
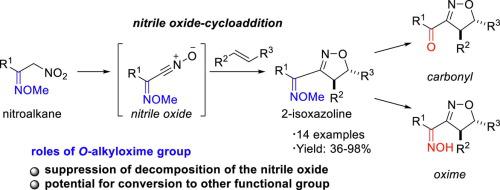
Nitrile oxides, which are easily generated in situ from widely available nitroalkanes, can be used to directly access 2-isoxazoline heterocycles through 1,3-dipolar cycloaddition reactions with alkenes. These heterocycles are not only useful core fragments in pharmaceutical and agricultural chemistry, but also valuable building blocks convertible to multifunctional groups, such as β-hydroxy ketones. Herein, we have developed nitrile oxide cycloaddition reactions between nitrile oxides derived from O-alkyloxime-substituted nitroalkanes and various alkenes. This methodology can be applied to construct 2-isoxazolines possessing electron-withdrawing groups, which are difficult to access using the conventional approach.
中文翻译:

烯烃或炔烃与硝基烷烃被可转化为各种官能团的O-烷基肟取代的一氧化氮环加成反应
易从易得的硝基烷烃中就地生成的腈氧化物可用于通过与烯烃的1,3-偶极环加成反应直接进入2-异恶唑啉杂环。这些杂环不仅是医药和农业化学中有用的核心片段,而且还是可转化为多功能基团(例如β-羟基酮)的有价值的构建基块。在本文中,我们已经开发了衍生自O-烷基肟取代的硝基烷烃的腈氧化物与各种烯烃之间的腈氧化物环加成反应。该方法可用于构建具有吸电子基团的2-异恶唑啉,使用常规方法难以接近。










































 京公网安备 11010802027423号
京公网安备 11010802027423号