Tetrahedron ( IF 2.1 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.tet.2020.131384 Hecham Aboubakr , Jean-Marc Sotiropoulos , Hugues Brisset , Jean-Manuel Raimundo
|
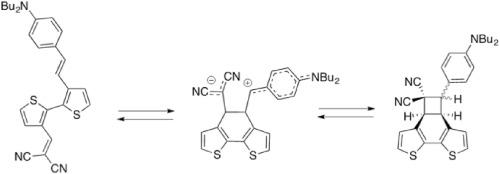
In the course of our studies of D-π-A push-pull chromophores we have envisioned the design of the novel 3-(4-N,N-dibutylamino)-styryl)-3’-(dicyanovinyl)-bithiophene derivative as a potential building block for optoelectronic applications. However, during the synthesis of this chromophore, a cycloadduct compound confirmed by X-ray structure was obtained by an intramolecular [2 + 2] cycloaddition reaction. The retro cycloaddition reaction carried out in thermal conditions and followed by UV–Visible absorption spectrophotometry afforded quantitively the target chromophore. Electrochemical properties recorded are in good agreement with open and close compounds. Mechanism involves cis/trans isomerization was proposed to explain the complete conversion.
中文翻译:

3-(4- N,N-二丁基氨基)-苯乙烯基)-3'-(二氰基乙烯基)-联噻吩可逆[2 + 2]环加成后的光学性质控制
在研究D-π-A推挽生色团的过程中,我们设想了将新型3-(4- N,N-二丁基氨基)-苯乙烯基)-3'-(二氰基乙烯基)-联噻吩衍生物设计为光电应用的潜在组成部分。然而,在该生色团的合成过程中,通过分子内[2 + 2]环加成反应获得了由X射线结构证实的环加合物。在热条件下进行逆向环加成反应,然后进行紫外可见吸收分光光度法定量地提供了目标生色团。记录的电化学性质与开放和封闭的化合物非常吻合。机理涉及顺式/反式异构化,用以解释完全转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号