Journal of Molecular Liquids ( IF 6 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.molliq.2020.113747 Ming Lin , Xianwei Hu , Jiangyu Yu , Youjian Yang , Zhongning Shi , Zhaowen Wang
|
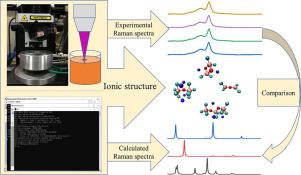
In-situ Raman spectroscopy and quantum mechanical calculations were used to study the forms of oxygen-containing complexes in molten NaF-AlF3 systems. The molar ratios of NaF to AlF3 were varied at 1.22, 1.9, and 2.7 at temperatures of 1023 K, 1223 K, and 1283 K, respectively. Upon adding alumina to molten NaF-AlF3 systems, oxygen-containing complexes, Al2OF4, Al3O2F83−, Al2OF84−, and Al2O2F42−, were formed. The structures of Al2OF4 and Na4Al2OF8 belong to the D2d and C2v point groups, respectively, while Na3Al3O2F8 and Na2Al2O2F4 structures belong to the C1 point group. The main bands in the experimental Raman spectra of Al2OF4, Al3O2F83−, Al2OF84−, and Al2O2F42− were located at 460 cm−1, 530 cm−1, 505 cm−1, and 400 cm−1, respectively. The types and contents of the oxygen-containing complex ions in the system were related to the NaF-to-AlF3 molar ratio and the concentration of alumina. In the molten NaF-AlF3-Al2O3 system, with a NaF-to-AlF3 molar ratio of 1.22, only Al2OF4 formed. When the molar ratio was increased to 1.9, the oxygen-containing entities generated were Al2OF4 and Al3O2F83−. The content of the two oxygen-containing entities was low when the alumina content was below 6 wt%. As the concentration of alumina was 6 wt% or more, Al3O2F83− gradually became the main oxygen-containing entity. For the system with a NaF-to-AlF3 molar ratio of 2.7, when the alumina concentration was less than 6 wt%, the main oxygen-containing entities were Al2OF4, Al3O2F83−, and Al2OF84−, but with low concentrations. The concentrations of Al3O2F83− and Al2OF84− ions increased considerably as the alumina content approached 6 wt%. Al2O2F42− was formed when the alumina concentration reached 10 wt%. The reaction equations occurring after adding alumina to molten NaF-AlF3 systems were also determined herein.
中文翻译:

用拉曼光谱和量子力学计算确定NaF-AlF 3系统中氧化铝的形貌
原位拉曼光谱和量子力学计算被用来研究熔融的NaF-AlF 3系统中含氧配合物的形式。NaF与AlF 3的摩尔比分别在1023 K,1223 K和1283 K的温度下变化为1.22、1.9和2.7。在将氧化铝添加到熔融的NaF-AlF 3体系中时,形成了含氧络合物Al 2 OF 4,Al 3 O 2 F 8 3-,Al 2 OF 8 4-和Al 2 O 2 F 4 2-。Al 2 OF的结构4和Na 4 Al 2 OF 8分别属于D2d和C2v点组,而Na 3 Al 3 O 2 F 8和Na 2 Al 2 O 2 F 4结构属于C1点组。Al 2 OF 4,Al 3 O 2 F 8 3−,Al 2 OF 8 4−和Al 2 O 2 F 4 2-的实验拉曼光谱中的主要谱带分别位于460厘米-1,530厘米-1,505厘米-1,和400厘米-1,分别。系统中含氧络合物离子的类型和含量与NaF / AlF 3摩尔比和氧化铝浓度有关。在熔融的NaF-AlF 3 -Al 2 O 3系统中,NaF与AlF 3的摩尔比为1.22,仅形成Al 2 OF 4。当摩尔比增加到1.9时,产生的含氧实体为Al 2 OF 4和Al 3 O 2 F 8。3−。当氧化铝含量低于6wt%时,两个含氧实体的含量低。当氧化铝的浓度为6wt%以上时,Al 3 O 2 F 8 3-逐渐成为主要的含氧实体。对于NaF / AlF 3摩尔比为2.7的系统,当氧化铝浓度小于6 wt%时,主要的含氧实体为Al 2 OF 4,Al 3 O 2 F 8 3-和Al 2 OF 8 4−,但浓度较低。Al 3 O 2 F的浓度随着氧化铝含量接近6 wt%,8 3-和Al 2 OF 8 4-离子显着增加。当氧化铝浓度达到10重量%时,形成Al 2 O 2 F 4 2-。在此还确定了在将氧化铝添加到熔融的NaF-AlF 3体系中之后发生的反应方程式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号