当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Narrow-Spectrum Antibiotic Targeting of the Radical SAM Enzyme MqnE in Menaquinone Biosynthesis.
Biochemistry ( IF 2.9 ) Pub Date : 2020-07-05 , DOI: 10.1021/acs.biochem.0c00070 Ayala G Carl 1 , Lawrence D Harris 2, 3 , Mu Feng 1 , Lars U Nordstrøm 1 , Gary J Gerfen 4 , Gary B Evans 2, 3 , Alexey Silakov 5 , Steven C Almo 1 , Tyler L Grove 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-07-05 , DOI: 10.1021/acs.biochem.0c00070 Ayala G Carl 1 , Lawrence D Harris 2, 3 , Mu Feng 1 , Lars U Nordstrøm 1 , Gary J Gerfen 4 , Gary B Evans 2, 3 , Alexey Silakov 5 , Steven C Almo 1 , Tyler L Grove 1
Affiliation
|
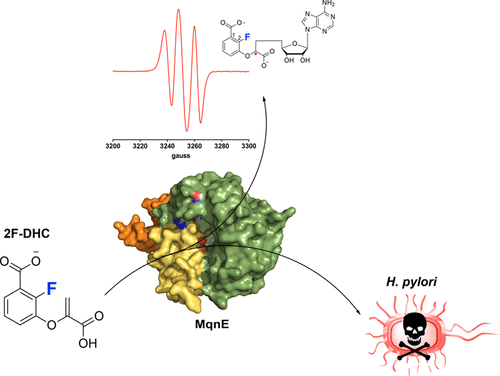
Antibiotic resistance continues to spread at an alarming rate, outpacing the introduction of new therapeutics and threatening to globally undermine health care. There is a crucial need for new strategies that selectively target specific pathogens while leaving the majority of the microbiome untouched, thus averting the debilitating and sometimes fatal occurrences of opportunistic infections. To address these challenges, we have adopted a unique strategy that focuses on oxygen-sensitive proteins, an untapped set of therapeutic targets. MqnE is a member of the radical S-adenosyl-l-methionine (RS) superfamily, all of which rely on an oxygen-sensitive [4Fe-4S] cluster for catalytic activity. MqnE catalyzes the conversion of didehydrochorismate to aminofutalosine in the essential menaquinone biosynthetic pathway present in a limited set of species, including the gut pathogen Helicobacter pylori (Hp), making it an attractive target for narrow-spectrum antibiotic development. Indeed, we show that MqnE is inhibited by the mechanism-derived 2-fluoro analogue of didehydrochorismate (2F-DHC) due to accumulation of a radical intermediate under turnover conditions. Structures of MqnE in the apo and product-bound states afford insight into its catalytic mechanism, and electron paramagnetic resonance approaches provide direct spectroscopic evidence consistent with the predicted structure of the radical intermediate. In addition, we demonstrate the essentiality of the menaquinone biosynthetic pathway and unambiguously validate 2F-DHC as a selective inhibitor of Hp growth that exclusively targets MqnE. These data provide the foundation for designing effective Hp therapies and demonstrate proof of principle that radical SAM proteins can be effectively leveraged as therapeutic targets.
中文翻译:

甲萘醌生物合成中自由基SAM酶MqnE的窄谱抗生素靶向。
抗生素耐药性继续以惊人的速度扩散,超过了新疗法的引入,并威胁到全球破坏卫生保健。迫切需要有针对性地选择针对特定病原体,同时又不影响大多数微生物组的新策略,从而避免机会性感染的衰弱和有时致命的发生。为了应对这些挑战,我们采用了一种独特的策略,重点研究对氧敏感的蛋白质,这是一组尚未开发的治疗靶标。MqnE是基团中的一员小号-adenosyl-升-蛋氨酸(RS)超家族,所有这些都依赖于对氧敏感的[4Fe-4S]簇来实现催化活性。MqnE在有限的一组物种(包括肠道病原体幽门螺杆菌(Hp))中存在的必需甲萘醌生物合成途径中,催化二氢胆酸酯转化为氨基富他肌酸),使其成为窄谱抗生素开发的有吸引力的目标。确实,我们表明,由于在翻转条件下自由基中间体的积累,MqnE受到机制的双脱氢胆酸(2-F-DHC)的2-氟类似物的抑制。载脂蛋白和产物结合态的MqnE结构提供了对其催化机理的深入了解,电子顺磁共振方法提供了与自由基中间体的预测结构一致的直接光谱证据。此外,我们证明了甲萘醌生物合成途径的必要性,并明确验证2F-DHC作为Hp生长的选择性抑制剂(专门针对MqnE)。这些数据为设计有效的Hp提供了基础。 疗法并证明了原理性证据,即自由基SAM蛋白可以有效地用作治疗靶标。
更新日期:2020-07-14
中文翻译:

甲萘醌生物合成中自由基SAM酶MqnE的窄谱抗生素靶向。
抗生素耐药性继续以惊人的速度扩散,超过了新疗法的引入,并威胁到全球破坏卫生保健。迫切需要有针对性地选择针对特定病原体,同时又不影响大多数微生物组的新策略,从而避免机会性感染的衰弱和有时致命的发生。为了应对这些挑战,我们采用了一种独特的策略,重点研究对氧敏感的蛋白质,这是一组尚未开发的治疗靶标。MqnE是基团中的一员小号-adenosyl-升-蛋氨酸(RS)超家族,所有这些都依赖于对氧敏感的[4Fe-4S]簇来实现催化活性。MqnE在有限的一组物种(包括肠道病原体幽门螺杆菌(Hp))中存在的必需甲萘醌生物合成途径中,催化二氢胆酸酯转化为氨基富他肌酸),使其成为窄谱抗生素开发的有吸引力的目标。确实,我们表明,由于在翻转条件下自由基中间体的积累,MqnE受到机制的双脱氢胆酸(2-F-DHC)的2-氟类似物的抑制。载脂蛋白和产物结合态的MqnE结构提供了对其催化机理的深入了解,电子顺磁共振方法提供了与自由基中间体的预测结构一致的直接光谱证据。此外,我们证明了甲萘醌生物合成途径的必要性,并明确验证2F-DHC作为Hp生长的选择性抑制剂(专门针对MqnE)。这些数据为设计有效的Hp提供了基础。 疗法并证明了原理性证据,即自由基SAM蛋白可以有效地用作治疗靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号