当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The D1:Ser268 residue of Photosystem II contributes to an alternative pathway for Q B protonation in the absence of bound bicarbonate
FEBS Letters ( IF 3.0 ) Pub Date : 2020-07-26 , DOI: 10.1002/1873-3468.13880 Jack A Forsman 1 , Julian J Eaton-Rye 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-07-26 , DOI: 10.1002/1873-3468.13880 Jack A Forsman 1 , Julian J Eaton-Rye 1
Affiliation
|
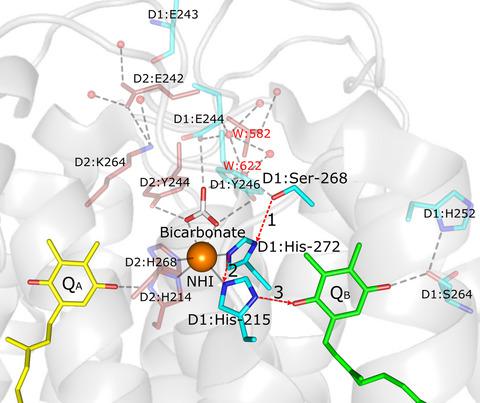
Photosystem II catalyses the splitting of water and the reduction in plastoquinone in thylakoid membranes of all oxygenic photosynthetic organisms. The final step in quinol formation is protonation of the reduced secondary quinone electron acceptor QB2‐(H+) to give QBH2. The proton for this step is hypothesized to be provided by a hydrogen‐bond network incorporating amino acids from the Photosystem II D1 and D2 reaction center proteins, together with several bound waters and a bicarbonate ion ligated to a non‐heme iron found between the primary plastoquinone electron acceptor QA and QB. The aim of this study was to investigate the role of bicarbonate and the D1:Ser268 residue in the formation of QBH2. Using targeted mutagenesis of the D1 protein in the cyanobacterium Synechocystis sp. PCC 6803, we have created two mutants, S268A and S268T. Our D1:Ser268 mutants exhibited increased sensitivity to formate‐induced inhibition of electron transfer between QA and QB and indicate that D1:Ser268 and bicarbonate support the second protonation in the formation of QBH2 via two different pathways that both lead to the protonation of QB2‐(H+) by D1:His215.
中文翻译:

在没有结合碳酸氢盐的情况下,光系统 II 的 D1:Ser268 残基有助于 QB 质子化的替代途径
光系统 II 催化所有含氧光合生物类囊体膜中水的分解和质体醌的还原。醌醇形成的最后一步是将还原的二级醌电子受体 QB2-(H+) 质子化,得到 QBH2。假设此步骤的质子由氢键网络提供,该网络包含来自光系统 II D1 和 D2 反应中心蛋白的氨基酸,以及几个结合水和一个碳酸氢根离子,该离子与初级之间发现的非血红素铁连接。质体醌电子受体 QA 和 QB。本研究的目的是研究碳酸氢盐和 D1:Ser268 残基在 QBH2 形成中的作用。在蓝藻集胞藻中使用 D1 蛋白的靶向诱变。PCC 6803,我们创建了两个突变体,S268A 和 S268T。我们的 D1:
更新日期:2020-07-26
中文翻译:

在没有结合碳酸氢盐的情况下,光系统 II 的 D1:Ser268 残基有助于 QB 质子化的替代途径
光系统 II 催化所有含氧光合生物类囊体膜中水的分解和质体醌的还原。醌醇形成的最后一步是将还原的二级醌电子受体 QB2-(H+) 质子化,得到 QBH2。假设此步骤的质子由氢键网络提供,该网络包含来自光系统 II D1 和 D2 反应中心蛋白的氨基酸,以及几个结合水和一个碳酸氢根离子,该离子与初级之间发现的非血红素铁连接。质体醌电子受体 QA 和 QB。本研究的目的是研究碳酸氢盐和 D1:Ser268 残基在 QBH2 形成中的作用。在蓝藻集胞藻中使用 D1 蛋白的靶向诱变。PCC 6803,我们创建了两个突变体,S268A 和 S268T。我们的 D1:











































 京公网安备 11010802027423号
京公网安备 11010802027423号