当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation of multiepitope cancer vaccines based on large combinatorial libraries of survivin-derived mutant epitopes.
Immunology ( IF 4.9 ) Pub Date : 2020-07-03 , DOI: 10.1111/imm.13233 Allan Noé Domínguez-Romero 1 , Fernando Martínez-Cortés 1 , María Elena Munguía 1 , Josué Odales 1 , Goar Gevorkian 1 , Karen Manoutcharian 1
Immunology ( IF 4.9 ) Pub Date : 2020-07-03 , DOI: 10.1111/imm.13233 Allan Noé Domínguez-Romero 1 , Fernando Martínez-Cortés 1 , María Elena Munguía 1 , Josué Odales 1 , Goar Gevorkian 1 , Karen Manoutcharian 1
Affiliation
|
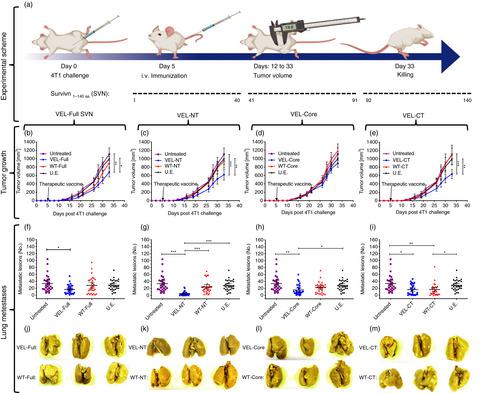
Immune tolerance is the main challenge in the field of cancer vaccines, so modified peptide sequences or naturally occurring mutated versions of cancer‐related wild‐type (WT) antigens represent a promising pathway. However, the low immunogenicity of mutation‐induced neoantigens and, particularly, their incapacity to activate CD8+ T cells are generating doubts on the success of neoantigen‐based cancer vaccines in clinical trials. We developed a novel vaccine approach based on a new class of vaccine immunogens, called variable epitope libraries (VELs). We used three regions of survivin (SVN), composed of 40, 49 and 51 amino acids, along with the complete SVN protein to generate the VELs as multiepitope vaccines. BALB/c mice, challenged with the aggressive and highly metastatic 4T1 cell line, were vaccinated in a therapeutic setting. We showed significant tumor growth inhibition and, most importantly, strong suppression of lung metastasis after a single immunization using VEL vaccines. We demonstrated vaccine‐induced broad cellular immune responses concomitant with extensive tumor infiltration of T cells, the activation of CD107a+ IFN‐γ+ T cells in the spleen and a significant increase in the number of CD3+ CD8+ Ly6C+ effector T cells. In addition, we observed the presence of interferon‐γ‐, granzyme B‐ and perforin‐producing lymphocytes along with modifications in the amount of CD11b+ Ly6Cint/low Ly6G+ granulocytic myeloid‐derived suppressor cells and CD4+ CD25+ FoxP3+ regulatory T cells in the lungs and tumors of mice. In summary, we showed that the VELs represent a potent new class of cancer immunotherapy and propose the application of the VEL vaccine concept as a true alternative to currently available vaccine platforms.
中文翻译:

基于生存素衍生突变表位的大型组合文库生成多表位癌症疫苗。
免疫耐受是癌症疫苗领域的主要挑战,因此修饰的肽序列或自然发生的癌症相关野生型(WT)抗原的突变版本代表了一条有前途的途径。然而,突变诱导的新抗原的低免疫原性,特别是它们无法激活 CD8 + T 细胞,使人们对基于新抗原的癌症疫苗在临床试验中的成功产生了怀疑。我们开发了一种基于新型疫苗免疫原的新型疫苗方法,称为可变表位库(VEL)。我们使用生存素 (SVN) 的三个区域(由 40、49 和 51 个氨基酸组成)以及完整的 SVN 蛋白来生成作为多表位疫苗的 VEL。BALB/c 小鼠接受侵袭性和高度转移性 4T1 细胞系的攻击,并在治疗环境中接种疫苗。使用 VEL 疫苗进行单次免疫后,我们表现出显着的肿瘤生长抑制作用,最重要的是,对肺转移的强烈抑制。我们证明了疫苗诱导的广泛细胞免疫反应,伴随着 T 细胞的广泛肿瘤浸润、脾脏中 CD107a + IFN- γ + T 细胞的激活以及 CD3 + CD8 + Ly6C +效应 T 细胞数量的显着增加。此外,我们观察到产生干扰素γ、颗粒酶 B 和穿孔素的淋巴细胞的存在,以及 CD11b + Ly6C int/低 Ly6G +粒细胞骨髓源性抑制细胞和 CD4 + CD25 + FoxP3 +调节细胞数量的变化。小鼠肺部和肿瘤中的 T 细胞。总之,我们表明 VEL 代表了一种有效的新型癌症免疫疗法,并提出应用 VEL 疫苗概念作为当前可用疫苗平台的真正替代方案。
更新日期:2020-07-03
中文翻译:

基于生存素衍生突变表位的大型组合文库生成多表位癌症疫苗。
免疫耐受是癌症疫苗领域的主要挑战,因此修饰的肽序列或自然发生的癌症相关野生型(WT)抗原的突变版本代表了一条有前途的途径。然而,突变诱导的新抗原的低免疫原性,特别是它们无法激活 CD8 + T 细胞,使人们对基于新抗原的癌症疫苗在临床试验中的成功产生了怀疑。我们开发了一种基于新型疫苗免疫原的新型疫苗方法,称为可变表位库(VEL)。我们使用生存素 (SVN) 的三个区域(由 40、49 和 51 个氨基酸组成)以及完整的 SVN 蛋白来生成作为多表位疫苗的 VEL。BALB/c 小鼠接受侵袭性和高度转移性 4T1 细胞系的攻击,并在治疗环境中接种疫苗。使用 VEL 疫苗进行单次免疫后,我们表现出显着的肿瘤生长抑制作用,最重要的是,对肺转移的强烈抑制。我们证明了疫苗诱导的广泛细胞免疫反应,伴随着 T 细胞的广泛肿瘤浸润、脾脏中 CD107a + IFN- γ + T 细胞的激活以及 CD3 + CD8 + Ly6C +效应 T 细胞数量的显着增加。此外,我们观察到产生干扰素γ、颗粒酶 B 和穿孔素的淋巴细胞的存在,以及 CD11b + Ly6C int/低 Ly6G +粒细胞骨髓源性抑制细胞和 CD4 + CD25 + FoxP3 +调节细胞数量的变化。小鼠肺部和肿瘤中的 T 细胞。总之,我们表明 VEL 代表了一种有效的新型癌症免疫疗法,并提出应用 VEL 疫苗概念作为当前可用疫苗平台的真正替代方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号