当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid-liquid equilibria for the ternary systems of RbX + CdX2 + H2O (X =Cl, Br) at T = 298.15 K and the thermodynamic properties of the new solid compounds
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106238 Zhan-Ping Qiao , Tian Yang , Shu-Zhi Cao , Yu-Feng Tang , Qi-Chao Yang
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106238 Zhan-Ping Qiao , Tian Yang , Shu-Zhi Cao , Yu-Feng Tang , Qi-Chao Yang
|
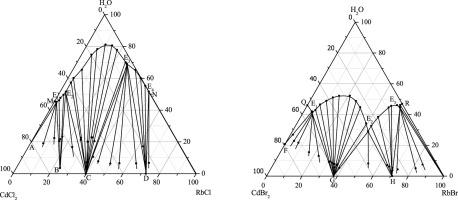
Abstract Solid-liquid equilibria in the ternary systems RbX + CdX2 + H2O (X =Cl, Br) at T = 298.15 K and under 101.3 kPa were investigated by the method of isothermal solution saturation, and the corresponding phase diagrams were constructed. It was found that there were five crystallization regions corresponding to CdCl2·2.5H2O, RbCd2Cl5·H2O, RbCdCl3, Rb4CdCl6 and RbCl in the system (RbCl + CdCl2 + H2O). Four crystallization regions corresponding to CdBr2·4H2O, RbCdBr3, Rb4CdBr6 and RbBr were found in the system (RbBr + CdBr2 + H2O). The new solid compounds RbCdCl3, RbCdBr3 and Rb4CdBr6 were congruently soluble in water, and new double salts RbCd2Cl5·H2O and Rb4CdCl6 were incongruently soluble in water. The new solid-phase compounds RbCd2Cl5·H2O, RbCdCl3, Rb4CdCl6, RbCdBr3 and Rb4CdBr6 were characterized by chemical analysis, XRD and TG-DTG techniques. The standard molar enthalpies of solution of RbCd2Cl5·H2O, RbCdCl3, Rb4CdCl6, RbCdBr3 and Rb4CdBr6 in water were measured to be (20.74 ± 0.16) kJ·mol-1, (28.00 ± 0.39) kJ·mol-1, (96.56 ± 0.50) kJ·mol-1, (35.38 ± 0.33) kJ·mol-1 and (110.64 ± 0.62) kJ·mol-1 by microcalorimetry in the condition of infinite dilution, and their standard molar enthalpies of formation were determined to be –(1545.3 ± 0.6) kJ·mol-1, –(856.5 ± 0.7) kJ·mol-1, –(2180.1 ± 1.3) kJ·mol-1, –(727.1 ± 0.7) kJ·mol-1 and –(1920.5 ± 1.3) kJ·mol-1, respectively.
中文翻译:

RbX + CdX2 + H2O (X = Cl, Br) 三元体系在 T = 298.15 K 时的固液平衡和新固体化合物的热力学性质
摘要 采用等温溶液饱和法研究了T = 298.15 K和101.3 kPa下三元体系RbX + CdX2 + H2O (X = Cl, Br)的固液平衡,并构建了相应的相图。发现体系中有五个结晶区分别对应于CdCl2·2.5H2O、RbCd2Cl5·H2O、RbCdCl3、Rb4CdCl6和RbCl(RbCl+CdCl2+H2O)。在体系中发现了对应于CdBr2·4H2O、RbCdBr3、Rb4CdBr6和RbBr的四个结晶区(RbBr+CdBr2+H2O)。新固体化合物RbCdCl3、RbCdBr3和Rb4CdBr6均溶于水,新复盐RbCd2Cl5·H2O和Rb4CdCl6不溶于水。通过化学分析对新的固相化合物RbCd2Cl5·H2O、RbCdCl3、Rb4CdCl6、RbCdBr3和Rb4CdBr6进行了表征,XRD 和 TG-DTG 技术。测得RbCd2Cl5·H2O、RbCdCl3、Rb4CdCl6、RbCdBr3和Rb4CdBr6在水中的标准摩尔焓为(20.74±0.16)kJ·mol-1,(28.00±0.39)kJ·6.50,(20.74±0.16)kJ·6.50, ) kJ·mol-1、(35.38±0.33)kJ·mol-1和(110.64±0.62)kJ·mol-1在无限稀释条件下微量热测定,其标准摩尔生成焓为-( 1545.3 ± 0.6) kJ·mol-1、–(856.5 ± 0.7) kJ·mol-1、–(2180.1 ± 1.3) kJ·mol-1、–(727.1 ± 0.7) kJ·mol-1 和–(1920.5 ± 1.3) kJ·mol-1,分别。
更新日期:2020-12-01
中文翻译:

RbX + CdX2 + H2O (X = Cl, Br) 三元体系在 T = 298.15 K 时的固液平衡和新固体化合物的热力学性质
摘要 采用等温溶液饱和法研究了T = 298.15 K和101.3 kPa下三元体系RbX + CdX2 + H2O (X = Cl, Br)的固液平衡,并构建了相应的相图。发现体系中有五个结晶区分别对应于CdCl2·2.5H2O、RbCd2Cl5·H2O、RbCdCl3、Rb4CdCl6和RbCl(RbCl+CdCl2+H2O)。在体系中发现了对应于CdBr2·4H2O、RbCdBr3、Rb4CdBr6和RbBr的四个结晶区(RbBr+CdBr2+H2O)。新固体化合物RbCdCl3、RbCdBr3和Rb4CdBr6均溶于水,新复盐RbCd2Cl5·H2O和Rb4CdCl6不溶于水。通过化学分析对新的固相化合物RbCd2Cl5·H2O、RbCdCl3、Rb4CdCl6、RbCdBr3和Rb4CdBr6进行了表征,XRD 和 TG-DTG 技术。测得RbCd2Cl5·H2O、RbCdCl3、Rb4CdCl6、RbCdBr3和Rb4CdBr6在水中的标准摩尔焓为(20.74±0.16)kJ·mol-1,(28.00±0.39)kJ·6.50,(20.74±0.16)kJ·6.50, ) kJ·mol-1、(35.38±0.33)kJ·mol-1和(110.64±0.62)kJ·mol-1在无限稀释条件下微量热测定,其标准摩尔生成焓为-( 1545.3 ± 0.6) kJ·mol-1、–(856.5 ± 0.7) kJ·mol-1、–(2180.1 ± 1.3) kJ·mol-1、–(727.1 ± 0.7) kJ·mol-1 和–(1920.5 ± 1.3) kJ·mol-1,分别。











































 京公网安备 11010802027423号
京公网安备 11010802027423号