Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-07-04 , DOI: 10.1016/j.saa.2020.118663 Marek Drozd 1
|
The noncentrosymmetric complex of guanidine with 4-nitro benzoic acid is known in the literature. The X-ray structure at room temperature was described.
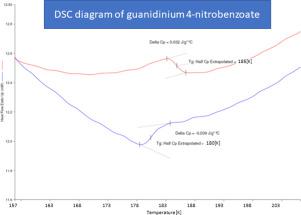
In our calorimetric (DSC) study the phase transition at ca. 183 K was detected. This phenomenon has a typical continuous (second-order) character. The DSC studies were expanded, and the deuterated sample was measured, too. During the deuteration process, the temperature of the phase transition is changed.
The detailed vibrational (infrared and Raman) studies were performed for normal and deuterated powder samples at room temperature.
The mechanism of a phase transition was studied by vibrational spectroscopy, also. A temperature dependence infrared spectra were measured in the temperature range 11–300 K. Additionally, the low-temperature spectra were measured for the deuterated sample. Based on these studies, the detailed assignments of observed bands were made.
中文翻译:

量热法和分光光度法研究了4-硝基苯甲酸胍盐的相变机理。
胍与4-硝基苯甲酸的非中心对称络合物在文献中是已知的。描述了室温下的X射线结构。
在我们的量热法(DSC)中,研究了相变点。检测到183K。此现象具有典型的连续(二阶)特征。扩展了DSC研究,还测量了氘代样品。在氘化过程中,相变温度会改变。
在室温下对正常和氘代粉末样品进行了详细的振动(红外和拉曼)研究。
还通过振动光谱研究了相变的机理。在11–300 K的温度范围内测量了温度依赖性红外光谱。此外,还对氘化样品进行了低温光谱测量。基于这些研究,对观察频带进行了详细分配。











































 京公网安备 11010802027423号
京公网安备 11010802027423号